当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A review on fundamentals for designing oxygen evolution electrocatalysts
Chemical Society Reviews ( IF 46.2 ) Pub Date : 2020-03-05 , DOI: 10.1039/c9cs00607a Jiajia Song 1, 2, 3, 4, 5 , Chao Wei 5, 6, 7, 8, 9 , Zhen-Feng Huang 6, 8, 10, 11 , Chuntai Liu 12, 13, 14, 15, 16 , Lin Zeng 16, 17, 18, 19 , Xin Wang 6, 8, 10, 11 , Zhichuan J. Xu 5, 6, 7, 8, 20
Chemical Society Reviews ( IF 46.2 ) Pub Date : 2020-03-05 , DOI: 10.1039/c9cs00607a Jiajia Song 1, 2, 3, 4, 5 , Chao Wei 5, 6, 7, 8, 9 , Zhen-Feng Huang 6, 8, 10, 11 , Chuntai Liu 12, 13, 14, 15, 16 , Lin Zeng 16, 17, 18, 19 , Xin Wang 6, 8, 10, 11 , Zhichuan J. Xu 5, 6, 7, 8, 20
Affiliation
|
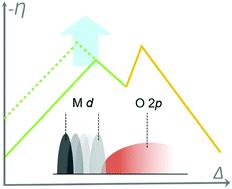
Electricity-driven water splitting can facilitate the storage of electrical energy in the form of hydrogen gas. As a half-reaction of electricity-driven water splitting, the oxygen evolution reaction (OER) is the major bottleneck due to the sluggish kinetics of this four-electron transfer reaction. Developing low-cost and robust OER catalysts is critical to solving this efficiency problem in water splitting. The catalyst design has to be built based on the fundamental understanding of the OER mechanism and the origin of the reaction overpotential. In this article, we summarize the recent progress in understanding OER mechanisms, which include the conventional adsorbate evolution mechanism (AEM) and lattice-oxygen-mediated mechanism (LOM) from both theoretical and experimental aspects. We start with the discussion on the AEM and its linked scaling relations among various reaction intermediates. The strategies to reduce overpotential based on the AEM and its derived descriptors are then introduced. To further reduce the OER overpotential, it is necessary to break the scaling relation of HOO* and HO* intermediates in conventional AEM to go beyond the activity limitation of the volcano relationship. Strategies such as stabilization of HOO*, proton acceptor functionality, and switching the OER pathway to LOM are discussed. The remaining questions on the OER and related perspectives are also presented at the end.
中文翻译:

析氧电催化剂设计基础的综述
电力驱动的水分解可以以氢气的形式促进电能的存储。作为电驱动水分解的半反应,由于这种四电子转移反应的动力学缓慢,因此析氧反应(OER)是主要瓶颈。开发低成本耐用的OER催化剂对于解决分水效率问题至关重要。催化剂的设计必须基于对OER机理的基本理解和反应超电势的起因。在本文中,我们从理论和实验两个方面总结了了解OER机理的最新进展,包括常规的吸附物逸出机理(AEM)和晶格氧介导的机理(LOM)。我们首先讨论AEM及其在各种反应中间体之间的关联比例关系。然后介绍了基于AEM及其派生描述符的减少过电势的策略。为了进一步降低OER超电势,有必要打破常规AEM中HOO *和HO *中间体的比例关系,使其超出火山关系的活动极限。讨论了诸如稳定HOO *,质子受体功能以及将OER途径转换为LOM之类的策略。最后,还将介绍有关OER和相关观点的其余问题。有必要打破常规AEM中HOO *和HO *中间体的比例关系,以超出火山关系的活动极限。讨论了诸如稳定HOO *,质子受体功能以及将OER途径转换为LOM之类的策略。最后,还将介绍有关OER和相关观点的其余问题。有必要打破常规AEM中HOO *和HO *中间体的比例关系,以超出火山关系的活动极限。讨论了诸如稳定HOO *,质子受体功能以及将OER途径转换为LOM之类的策略。最后,还将介绍有关OER和相关观点的其余问题。
更新日期:2020-04-24
中文翻译:

析氧电催化剂设计基础的综述
电力驱动的水分解可以以氢气的形式促进电能的存储。作为电驱动水分解的半反应,由于这种四电子转移反应的动力学缓慢,因此析氧反应(OER)是主要瓶颈。开发低成本耐用的OER催化剂对于解决分水效率问题至关重要。催化剂的设计必须基于对OER机理的基本理解和反应超电势的起因。在本文中,我们从理论和实验两个方面总结了了解OER机理的最新进展,包括常规的吸附物逸出机理(AEM)和晶格氧介导的机理(LOM)。我们首先讨论AEM及其在各种反应中间体之间的关联比例关系。然后介绍了基于AEM及其派生描述符的减少过电势的策略。为了进一步降低OER超电势,有必要打破常规AEM中HOO *和HO *中间体的比例关系,使其超出火山关系的活动极限。讨论了诸如稳定HOO *,质子受体功能以及将OER途径转换为LOM之类的策略。最后,还将介绍有关OER和相关观点的其余问题。有必要打破常规AEM中HOO *和HO *中间体的比例关系,以超出火山关系的活动极限。讨论了诸如稳定HOO *,质子受体功能以及将OER途径转换为LOM之类的策略。最后,还将介绍有关OER和相关观点的其余问题。有必要打破常规AEM中HOO *和HO *中间体的比例关系,以超出火山关系的活动极限。讨论了诸如稳定HOO *,质子受体功能以及将OER途径转换为LOM之类的策略。最后,还将介绍有关OER和相关观点的其余问题。



























 京公网安备 11010802027423号
京公网安备 11010802027423号