PLOS Biology ( IF 7.8 ) Pub Date : 2020-03-05 , DOI: 10.1371/journal.pbio.3000654 Hongxin Guan 1 , Youwang Wang 2, 3 , Ting Yu 1 , Yini Huang 1, 4 , Mianhuan Li 1, 5 , Abdullah F U H Saeed 1 , Vanja Perčulija 1 , Daliang Li 1 , Jia Xiao 1, 5 , Dongmei Wang 1 , Ping Zhu 2, 3 , Songying Ouyang 1, 2, 4
|
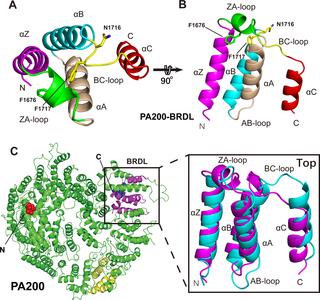
Proteasomes are highly abundant and conserved protease complexes that eliminate unwanted proteins in the cells. As a single-chain ATP-independent nuclear proteasome activator, proteasome activator 200 (PA200) associates with 20S core particle to form proteasome complex that catalyzes polyubiquitin-independent degradation of acetylated histones, thus playing a pivotal role in DNA repair and spermatogenesis. Here, we present cryo–electron microscopy (cryo-EM) structures of the human PA200-20S complex and PA200 at 2.72 Å and 3.75 Å, respectively. PA200 exhibits a dome-like architecture that caps 20S and uses its C-terminal YYA (Tyr-Tyr-Ala) to induce the α-ring rearrangements and partial opening of the 20S gate. Our structural data also indicate that PA200 has two openings formed by numerous positively charged residues that respectively bind (5,6)-bisdiphosphoinositol tetrakisphosphate (5,6[PP]2-InsP4) and inositol hexakisphosphate (InsP6) and are likely to be the gates that lead unfolded proteins through PA200 and into the 20S. Besides, our structural analysis of PA200 found that the bromodomain (BRD)-like (BRDL) domain of PA200 shows considerable sequence variation in comparison to other human BRDs, as it contains only 82 residues because of a short ZA loop, and cannot be classified into any of the eight typical human BRD families. Taken together, the results obtained from this study provide important insights into human PA200-induced 20S gate opening for substrate degradation and the opportunities to explore the mechanism for its recognition of H4 histone in acetylation-mediated proteasomal degradation.
中文翻译:

人类PA200和PA200-20S复合体的低温电磁结构揭示了蛋白酶体门打开和两个PA200孔径的调节。
蛋白酶体是高度丰富且保守的蛋白酶复合物,可消除细胞中不需要的蛋白质。作为不依赖单链ATP的核蛋白酶体活化剂,蛋白酶体活化剂200(PA200)与20S核心颗粒结合形成蛋白酶体复合物,该复合物催化乙酰素组蛋白的多泛素非依赖性降解,从而在DNA修复和精子形成中发挥关键作用。在这里,我们介绍人类PA200-20S复合体和PA200分别在2.72Å和3.75Å时的低温电子显微镜(cryo-EM)结构。PA200呈现出一个圆顶状的结构,该结构覆盖20S并使用其C端YYA(Tyr-Tyr-Ala)引起α环重排和20S栅极的部分打开。我们的结构数据还表明,PA200具有两个分别由大量带正电荷的残基形成的开口,这些残基分别结合(5,4)和六磷酸肌醇(InsP 6),很可能是导致未折叠蛋白通过PA200进入20S的大门。此外,我们对PA200的结构分析发现,与其他人类BRD相比,PA200的溴结构域(BRD)样(BRDL)结构域显示出相当大的序列变异,因为它的ZA环很短,仅包含82个残基,因此无法分类进入八个典型人类BRD家族中的任何一个。两者合计,从这项研究中获得的结果提供了重要的见解,为人类PA200诱导的20S门打开的底物降解和探索其识别乙酰化介导的蛋白酶体降解中H4组蛋白的机制的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号