Nature Communications ( IF 16.6 ) Pub Date : 2020-03-05 , DOI: 10.1038/s41467-020-15048-8 Alexandra Rehn 1, 2 , Jannis Lawatscheck 1 , Marie-Lena Jokisch 1, 3 , Sophie L Mader 1 , Qi Luo 1, 4 , Franziska Tippel 1, 5 , Birgit Blank 1, 6 , Klaus Richter 1 , Kathrin Lang 1, 3 , Ville R I Kaila 1, 7 , Johannes Buchner 1
|
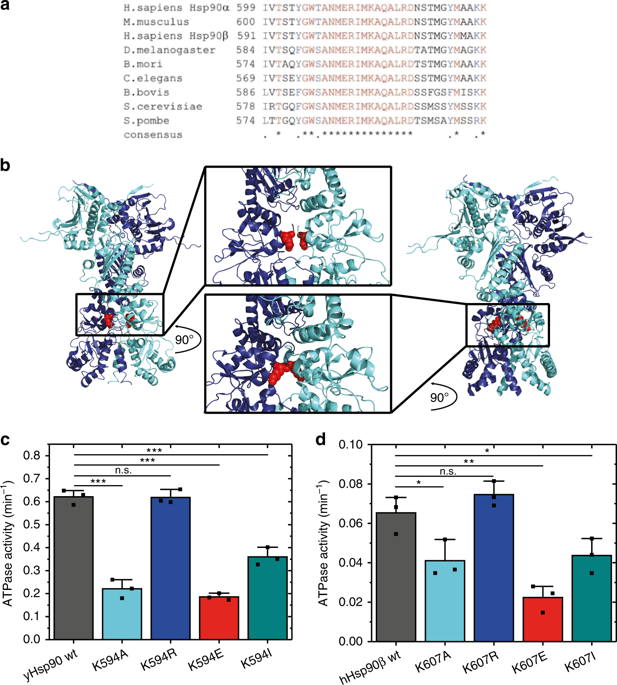
Methylation of a conserved lysine in C-terminal domain of the molecular chaperone Hsp90 was shown previously to affect its in vivo function. However, the underlying mechanism remained elusive. Through a combined experimental and computational approach, this study shows that this site is very sensitive to sidechain modifications and crucial for Hsp90 activity in vitro and in vivo. Our results demonstrate that this particular lysine serves as a switch point for the regulation of Hsp90 functions by influencing its conformational cycle, ATPase activity, co-chaperone regulation, and client activation of yeast and human Hsp90. Incorporation of the methylated lysine via genetic code expansion specifically shows that upon modification, the conformational cycle of Hsp90 is altered. Molecular dynamics simulations including the methylated lysine suggest specific conformational changes that are propagated through Hsp90. Thus, methylation of the C-terminal lysine allows a precise allosteric tuning of Hsp90 activity via long distances.
中文翻译:

甲基化的赖氨酸是伴侣蛋白Hsp90中构象通讯的转换点。
先前显示分子伴侣Hsp90的C末端结构域中的保守赖氨酸的甲基化会影响其体内功能。但是,基本机制仍然难以捉摸。通过组合的实验和计算方法,该研究表明该位点对侧链修饰非常敏感,对于体外和体内的Hsp90活性至关重要。我们的结果表明,这种特定的赖氨酸通过影响Hsp90的构象周期,ATPase活性,伴侣伴侣调节以及酵母和人Hsp90的客户激活而成为调节Hsp90功能的切入点。通过遗传密码扩展并入甲基化的赖氨酸,具体表明修饰后,Hsp90的构象循环发生了改变。包括甲基化赖氨酸在内的分子动力学模拟表明,特定的构象变化会通过Hsp90传播。因此,C末端赖氨酸的甲基化允许Hsp90活性通过长距离进行精确的变构调节。



























 京公网安备 11010802027423号
京公网安备 11010802027423号