Biochimie ( IF 3.3 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.biochi.2020.03.002 Suchitra Pradhan , Swathy Velaparambil Bipinachandran , Pratibha Kumari , M. Suguna , M. Dharma Prasad , Ravi Kumar
|
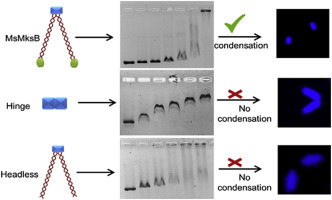
The structural maintenance of chromosomes (SMC) proteins play a vital role in genome stability and chromosome organization in all domains of life. Previous reports show that smc deletion causes decondensation of chromosome and an increased frequency of anucleated cells in bacteria. However, smc deletion in both Mycobacterium smegmatis and Mycobacterium tuberculosis did not affect chromosome condensation or the frequency of anucleated cells. In an attempt to understand this difference in M. smegmatis, we investigated the function of MksB (MsMksB), an alternate SMC-like protein. Like other bacterial SMCs, MsMksB is also an elongated homodimer, in which a central hinge domain connects two globular ATPase head domains via two coiled-coil arms. We show that full-length MsMksB binds to different topological forms of DNA without any preferences. However, the hinge and headless domains prefer binding to single-stranded DNA (ssDNA) and linear double-stranded DNA (dsDNA), respectively. The binding of MsMksB to DNA was independent of ATP as its ATP hydrolysis deficient mutant was also proficient in DNA binding. Further, the cytological profiling studies revealed that only the full-length MsMksB and none of its structural domains could condense the bacterial chromosome. This observation indicates the plausibility of the concerted action of different structural domains of SMC to bind and condense the chromosome. Moreover, MsMksB exhibited DNA stimulated ATPase activity, in addition to its intrinsic ATPase activity. Taken together, we have elucidated the function of an alternate bacterial condensin protein MksB and its structural domains in DNA binding and condensation.
中文翻译:

MksB,耻垢分枝杆菌的另一种凝缩素参与DNA结合和凝缩
染色体(SMC)蛋白质的结构维持在生命的所有领域中对基因组稳定性和染色体组织起着至关重要的作用。以前的报道表明,smc缺失会导致染色体的缩合和细菌中无核细胞的频率增加。但是,耻垢分枝杆菌和结核分枝杆菌中的smc缺失均不影响染色体浓缩或无核细胞的频率。为了了解耻垢分枝杆菌的这种差异,我们调查了MksB(MsMksB)(一种替代的SMC样蛋白)的功能。像其他细菌SMCs一样,MsMksB也是一种细长的同型二聚体,其中中央铰链结构域通过两个盘绕线圈臂连接两个球状ATPase头部结构域。我们显示全长MsMksB绑定到DNA的不同拓扑形式而没有任何偏好。但是,铰链和无头结构域更喜欢分别与单链DNA(ssDNA)和线性双链DNA(dsDNA)结合。MsMksB与DNA的结合独立于ATP,因为它的ATP水解缺陷型突变体也擅长DNA结合。此外,细胞学分析研究表明,只有全长MsMksB及其结构域都不能凝结细菌染色体。该观察结果表明SMC的不同结构域的协同作用结合和浓缩染色体的合理性。此外,MsMksB除了其固有的ATPase活性外,还具有DNA刺激的ATPase活性。两者合计,我们已经阐明了替代细菌凝聚素蛋白MksB的功能及其在DNA结合和缩合中的结构域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号