当前位置:
X-MOL 学术
›
Colloids Surf. B Biointerfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of thermal treatments on the structural change and the hemostatic property of hair extracted proteins.
Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.colsurfb.2020.110951 Kai-Chiang Yang , Lu-Ping Huang , Mao-Cong Huang , Aby A. Thyparambil , Yang Wei
Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.colsurfb.2020.110951 Kai-Chiang Yang , Lu-Ping Huang , Mao-Cong Huang , Aby A. Thyparambil , Yang Wei
|
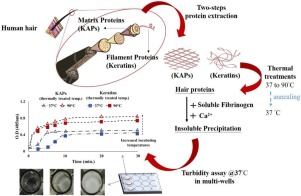
Human hair is a readily available source for hair protein-based biomaterial and is increasingly explored as an alternative to existing hemostatic materials. The hair protein is a complex mixture of multiple proteins, which are preferably extracted at relatively high temperatures (50-90 °C) for increasing protein yields. However, the effect of processing temperature on the hemostatic property of the hair derived proteins are not yet well-understood. The objective of the current study was to characterize the influence of thermal treatments (37 °C, 50 °C, 75 °C, 80 °C, and 90 °C) on the (i) secondary structure of different fractions of hair proteins including keratin (40-65 kDa) and keratin-associated proteins (KAPs, 6-30 kDa), and (ii) their capability to precipitate the soluble fibrinogen in an in vitro fibrin clotting assay. Our results indicated that the thermal treatments induced changes to the helical contents of hair-derived protein extracts and also increased the precipitation amount and rate of soluble fibrinogen. While further studies are required to better understand the exact role of hair protein fractions on the coagulation process, the current research suggests that the hair proteins extracted under relatively high temperatures is a prerequisite approach for improving the hemostatic property of human hair-derived proteins.
中文翻译:

热处理对毛发提取蛋白的结构变化和止血特性的影响。
人发是基于头发蛋白的生物材料的容易获得的来源,并且作为现有止血材料的替代物,人们的发掘越来越多。头发蛋白质是多种蛋白质的复杂混合物,为了提高蛋白质产量,最好在较高的温度(50-90°C)下提取。然而,加工温度对头发衍生的蛋白质的止血性能的影响尚未被很好地理解。当前研究的目的是表征热处理(37°C,50°C,75°C,80°C和90°C)对(i)头发蛋白不同部分的二级结构的影响,包括角蛋白(40-65 kDa)和角蛋白相关蛋白(KAPs,6-30 kDa),以及(ii)在体外血纤蛋白凝结测定中沉淀可溶性血纤蛋白原的能力。我们的结果表明,热处理引起了头发衍生的蛋白质提取物的螺旋含量的变化,并且还增加了可溶性纤维蛋白原的沉淀量和速率。虽然需要进一步的研究以更好地理解头发蛋白组分在凝血过程中的确切作用,但当前的研究表明,在相对较高的温度下提取的头发蛋白是改善人头发衍生蛋白的止血性能的前提方法。
更新日期:2020-03-05
中文翻译:

热处理对毛发提取蛋白的结构变化和止血特性的影响。
人发是基于头发蛋白的生物材料的容易获得的来源,并且作为现有止血材料的替代物,人们的发掘越来越多。头发蛋白质是多种蛋白质的复杂混合物,为了提高蛋白质产量,最好在较高的温度(50-90°C)下提取。然而,加工温度对头发衍生的蛋白质的止血性能的影响尚未被很好地理解。当前研究的目的是表征热处理(37°C,50°C,75°C,80°C和90°C)对(i)头发蛋白不同部分的二级结构的影响,包括角蛋白(40-65 kDa)和角蛋白相关蛋白(KAPs,6-30 kDa),以及(ii)在体外血纤蛋白凝结测定中沉淀可溶性血纤蛋白原的能力。我们的结果表明,热处理引起了头发衍生的蛋白质提取物的螺旋含量的变化,并且还增加了可溶性纤维蛋白原的沉淀量和速率。虽然需要进一步的研究以更好地理解头发蛋白组分在凝血过程中的确切作用,但当前的研究表明,在相对较高的温度下提取的头发蛋白是改善人头发衍生蛋白的止血性能的前提方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号