当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold nanobipyramid-loaded black phosphorus nanosheets for plasmon-enhanced photodynamic and photothermal therapy of deep-seated orthotopic lung tumors.
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.actbio.2020.03.001 Jing Wang 1 , Han Zhang 2 , Xiao Xiao 1 , Dong Liang 1 , Xinyue Liang 3 , Lan Mi 3 , Jianfang Wang 2 , Jun Liu 4
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.actbio.2020.03.001 Jing Wang 1 , Han Zhang 2 , Xiao Xiao 1 , Dong Liang 1 , Xinyue Liang 3 , Lan Mi 3 , Jianfang Wang 2 , Jun Liu 4
Affiliation
|
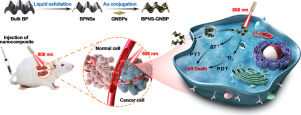
Various types of photodynamic agents have been explored for photodynamic therapy (PDT) to destroy cancers located in deep tissues. However, these agents are generally limited by low singlet oxygen (1O2) yields owing to weak absorption in the optical transparent window of biological tissues. Accordingly, in this work, we developed a nanocomposite through the assembly of gold nanobipyramids (GNBPs) on black phosphorus nanosheets (BPNSs). This nanocomposite could simultaneously enhance 1O2 generation and hyperthermia by localized surface plasmon resonance in cancer therapy. As two-dimensional inorganic photosensitizers, BPNSs were hybridized with GNBPs to form BPNS-GNBP hybrid nanosheets. The hybridization markedly increased 1O2 production by the BPNSs through plasmon-enhanced light absorption. The nanocomposite exhibited a higher photothermal conversion efficiency than the BPNSs alone. In vitro and in vivo assays indicated that the BPNS-GNBP hybrid nanocomposite exhibited good tumor inhibition efficacy owing to simultaneous dual-modality phototherapy. In vivo, the nanocomposite suppressed deep-seated tumor growth with minimal adverse effects in mice bearing orthotopic A549 human lung tumors. Taken together, these results demonstrated that our BPNS-GNBP nanocomposite could function as a promising dual-modality phototherapeutic agent for enhanced cancer therapy in future cancer treatments. STATEMENT OF SIGNIFICANCE: In this study, we established a new nanocomposite by assembly of gold nanobipyramids (GNBPs) on black phosphorus nanosheets (BPNSs). Characterization of this nanocomposite showed that BPNS-GNBP enhanced 1O2 generation and hyperthermia. BPNS-GNBP exhibited good tumor inhibition efficacy in vivo and in vitro owing to simultaneous dual-modal phototherapy functions. Moreover, BPNS-GNBP suppressed deep-seated tumor growth in vivo and did not show adverse effects in mice bearing orthotopic A549 human lung tumors. Overall, these results showed that BPNS-GNBP may be used as a promising dual-modal phototherapeutic agent for enhanced cancer therapy in future clinical applications.
中文翻译:

载有金纳米双金字塔的黑磷纳米片用于等离子增强深部原位肺肿瘤的光动力和光热疗法。
已经探索了各种类型的光动力剂用于光动力疗法(PDT),以破坏位于深部组织中的癌症。然而,由于在生物组织的光学透明窗中的吸收较弱,这些试剂通常受到单线态氧(1O2)产量低的限制。因此,在这项工作中,我们通过在黑磷纳米片(BPNSs)上组装金纳米双锥体(GNBPs)开发了一种纳米复合材料。这种纳米复合材料可以通过局部表面等离子体共振在癌症治疗中同时增强1O2的产生和高温。作为二维无机光敏剂,BPNS与GNBP杂交形成BPNS-GNBP杂化纳米片。通过等离激元增强的光吸收,杂交显着增加了BPNS产生的1O2产量。纳米复合材料显示出比单独的BPNS更高的光热转化效率。体外和体内试验表明,由于同时双模式光疗,BPNS-GNBP杂化纳米复合材料表现出良好的肿瘤抑制功效。在体内,该纳米复合材料在患有原位A549人肺肿瘤的小鼠中抑制了根深蒂固的肿瘤生长,并将不良反应降至最低。综上所述,这些结果表明,我们的BPNS-GNBP纳米复合材料可以作为一种有前途的双模态光疗剂,在未来的癌症治疗中增强癌症的治疗作用。重大意义声明:在这项研究中,我们通过在黑磷纳米片(BPNSs)上组装金纳米双锥体(GNBPs)建立了一种新的纳米复合材料。该纳米复合材料的表征表明,BPNS-GNBP增强了1O2的产生和热疗。由于同时具有双重模式的光疗功能,BPNS-GNBP在体内和体外均表现出良好的肿瘤抑制功效。此外,BPNS-GNBP在体内抑制了深层肿瘤的生长,并且在患有原位A549人肺肿瘤的小鼠中未显示出不良反应。总体而言,这些结果表明,BPNS-GNBP可用作未来临床应用中增强癌症治疗的有前途的双峰光疗剂。
更新日期:2020-03-05
中文翻译:

载有金纳米双金字塔的黑磷纳米片用于等离子增强深部原位肺肿瘤的光动力和光热疗法。
已经探索了各种类型的光动力剂用于光动力疗法(PDT),以破坏位于深部组织中的癌症。然而,由于在生物组织的光学透明窗中的吸收较弱,这些试剂通常受到单线态氧(1O2)产量低的限制。因此,在这项工作中,我们通过在黑磷纳米片(BPNSs)上组装金纳米双锥体(GNBPs)开发了一种纳米复合材料。这种纳米复合材料可以通过局部表面等离子体共振在癌症治疗中同时增强1O2的产生和高温。作为二维无机光敏剂,BPNS与GNBP杂交形成BPNS-GNBP杂化纳米片。通过等离激元增强的光吸收,杂交显着增加了BPNS产生的1O2产量。纳米复合材料显示出比单独的BPNS更高的光热转化效率。体外和体内试验表明,由于同时双模式光疗,BPNS-GNBP杂化纳米复合材料表现出良好的肿瘤抑制功效。在体内,该纳米复合材料在患有原位A549人肺肿瘤的小鼠中抑制了根深蒂固的肿瘤生长,并将不良反应降至最低。综上所述,这些结果表明,我们的BPNS-GNBP纳米复合材料可以作为一种有前途的双模态光疗剂,在未来的癌症治疗中增强癌症的治疗作用。重大意义声明:在这项研究中,我们通过在黑磷纳米片(BPNSs)上组装金纳米双锥体(GNBPs)建立了一种新的纳米复合材料。该纳米复合材料的表征表明,BPNS-GNBP增强了1O2的产生和热疗。由于同时具有双重模式的光疗功能,BPNS-GNBP在体内和体外均表现出良好的肿瘤抑制功效。此外,BPNS-GNBP在体内抑制了深层肿瘤的生长,并且在患有原位A549人肺肿瘤的小鼠中未显示出不良反应。总体而言,这些结果表明,BPNS-GNBP可用作未来临床应用中增强癌症治疗的有前途的双峰光疗剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号