当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Active S2168 and inactive S21IRS pinholin interact differently with the lipid bilayer: A 31P and 2H solid state NMR study.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.bbamem.2020.183257 Daniel L Drew 1 , Brandon Butcher 1 , Indra D Sahu 2 , Tanbir Ahammad 1 , Gunjan Dixit 1 , Gary A Lorigan 1
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.bbamem.2020.183257 Daniel L Drew 1 , Brandon Butcher 1 , Indra D Sahu 2 , Tanbir Ahammad 1 , Gunjan Dixit 1 , Gary A Lorigan 1
Affiliation
|
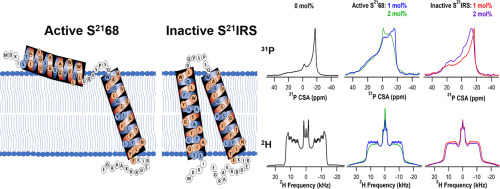
Pinholins are a family of lytic membrane proteins responsible for the lysis of the cytosolic membrane in host cells of double stranded DNA bacteriophages. Protein-lipid interactions have been shown to influence membrane protein topology as well as its function. This work investigated the interactions of pinholin with the phospholipid bilayer while in active and inactive confirmations to elucidate the different interactions the two forms have with the bilayer. Pinholin incorporated into deuterated DMPC-d54 lipid bilayers, along with 31P and 2H solid state NMR (SS-NMR) spectroscopy were used to probe the protein-lipid interactions with the phosphorus head group at the surface of the bilayer while interactions with the 2H nuclei were used to study the hydrophobic core. A comparison of the 31P chemical shift anisotropy (CSA) values of the active S2168 pinholin and inactive S21IRS pinholin indicated stronger head group interactions for the pinholin in its active form when compared to that of the inactive form supporting the model of a partially externalized peripheral transmembrane domain (TMD) of the active S2168 instead of complete externalized TMD1 as suggested by Ahammad et al. JPC B 2019. The 2H quadrupolar splitting analysis showed a decrease in spectral width for both forms of the pinholin when compared to the empty bilayers at all temperatures. In this case the decrease in the spectral width of the inactive S21IRS form of the pinholin showed stronger interactions with the acyl chains of the bilayer. The presence of the inactive form's additional TMD within the membrane was supported by the loss of peak resolution observed in the 2H NMR spectra.
中文翻译:

活性S2168和非活性S21IRS品醇与脂质双层的相互作用不同:一项31P和2H固态NMR研究。
Pinholins是一类裂解膜蛋白家族,负责双链DNA噬菌体宿主细胞中胞质膜的裂解。已经显示蛋白质-脂质相互作用会影响膜蛋白质拓扑及其功能。这项工作研究了品醇溶蛋白与磷脂双层的相互作用,同时在有活性和无活性的证实中阐明了两种形式与双层的不同相互作用。掺入氘化DMPC-d54脂质双层中的品醇结合31P和2H固态NMR(SS-NMR)光谱用于探测与双层表面磷头基团的蛋白质-脂质相互作用以及与2H核的相互作用被用来研究疏水核。活性S2168品醇和失活的S21IRS品醇的31P化学位移各向异性(CSA)值的比较表明,与支持部分外在化外周膜模型的非活性形式相比,品醇在其活性形式中的头部基团相互作用更强主动S2168的结构域(TMD),而不是Ahammad等人建议的完全外部化的TMD1。JPC B2019。与在所有温度下的空双层相比,两种形式的品醇的光谱宽度降低均显示在2H四极分裂分析中。在这种情况下,失活的松醇的S21IRS形式的光谱宽度的减小显示出与双层的酰基链的更强相互作用。无效表格的存在”
更新日期:2020-03-19
中文翻译:

活性S2168和非活性S21IRS品醇与脂质双层的相互作用不同:一项31P和2H固态NMR研究。
Pinholins是一类裂解膜蛋白家族,负责双链DNA噬菌体宿主细胞中胞质膜的裂解。已经显示蛋白质-脂质相互作用会影响膜蛋白质拓扑及其功能。这项工作研究了品醇溶蛋白与磷脂双层的相互作用,同时在有活性和无活性的证实中阐明了两种形式与双层的不同相互作用。掺入氘化DMPC-d54脂质双层中的品醇结合31P和2H固态NMR(SS-NMR)光谱用于探测与双层表面磷头基团的蛋白质-脂质相互作用以及与2H核的相互作用被用来研究疏水核。活性S2168品醇和失活的S21IRS品醇的31P化学位移各向异性(CSA)值的比较表明,与支持部分外在化外周膜模型的非活性形式相比,品醇在其活性形式中的头部基团相互作用更强主动S2168的结构域(TMD),而不是Ahammad等人建议的完全外部化的TMD1。JPC B2019。与在所有温度下的空双层相比,两种形式的品醇的光谱宽度降低均显示在2H四极分裂分析中。在这种情况下,失活的松醇的S21IRS形式的光谱宽度的减小显示出与双层的酰基链的更强相互作用。无效表格的存在”











































 京公网安备 11010802027423号
京公网安备 11010802027423号