当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeted In Situ Protein Diversification and Intra-organelle Validation in Mammalian Cells.
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.chembiol.2020.02.004 Mutlu Erdogan 1 , Arne Fabritius 1 , Jérome Basquin 2 , Oliver Griesbeck 1
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.chembiol.2020.02.004 Mutlu Erdogan 1 , Arne Fabritius 1 , Jérome Basquin 2 , Oliver Griesbeck 1
Affiliation
|
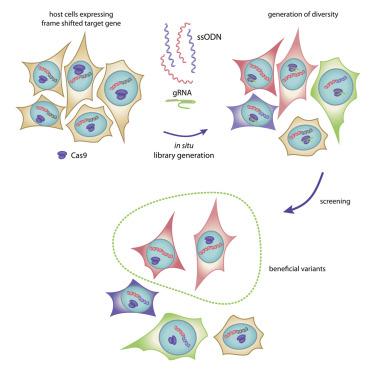
Engineered proteins must be phenotypically selected for function in the appropriate physiological context. Here, we present a versatile approach that allows generating panels of mammalian cells that express diversified heterologous protein libraries in the cytosol or subcellular compartments under stable conditions and in a single-variant-per-cell manner. To this end we adapt CRISPR/Cas9 editing technology to diversify targeted stretches of a protein of interest in situ. We demonstrate the utility of the approach by in situ engineering and intra-lysosome specific selection of an extremely pH-resistant long Stokes shift red fluorescent protein variant. Tailoring properties to specific conditions of cellular sub-compartments or organelles of mammalian cells can be an important asset to optimize various proteins, protein-based tools, and biosensors for distinct functions.
中文翻译:

哺乳动物细胞中的靶向原位蛋白质多样化和细胞器内验证。
工程蛋白质必须经过表型选择才能在适当的生理背景下发挥作用。在这里,我们提出了一种多功能方法,允许生成哺乳动物细胞组,这些细胞在稳定条件下以每细胞单一变体的方式在胞浆或亚细胞区室中表达多样化的异源蛋白质文库。为此,我们采用 CRISPR/Cas9 编辑技术,使感兴趣的蛋白质的目标片段原位多样化。我们通过原位工程和溶酶体内特异性选择极其耐 pH 的长斯托克斯位移红色荧光蛋白变体来证明该方法的实用性。根据哺乳动物细胞的细胞亚区室或细胞器的特定条件定制特性可能是优化各种蛋白质、基于蛋白质的工具和生物传感器以实现不同功能的重要资产。
更新日期:2020-03-05
中文翻译:

哺乳动物细胞中的靶向原位蛋白质多样化和细胞器内验证。
工程蛋白质必须经过表型选择才能在适当的生理背景下发挥作用。在这里,我们提出了一种多功能方法,允许生成哺乳动物细胞组,这些细胞在稳定条件下以每细胞单一变体的方式在胞浆或亚细胞区室中表达多样化的异源蛋白质文库。为此,我们采用 CRISPR/Cas9 编辑技术,使感兴趣的蛋白质的目标片段原位多样化。我们通过原位工程和溶酶体内特异性选择极其耐 pH 的长斯托克斯位移红色荧光蛋白变体来证明该方法的实用性。根据哺乳动物细胞的细胞亚区室或细胞器的特定条件定制特性可能是优化各种蛋白质、基于蛋白质的工具和生物传感器以实现不同功能的重要资产。











































 京公网安备 11010802027423号
京公网安备 11010802027423号