Cell ( IF 45.5 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.cell.2020.02.010 Yuka Nakazawa , Yuichiro Hara , Yasuyoshi Oka , Okiru Komine , Diana van den Heuvel , Chaowan Guo , Yasukazu Daigaku , Mayu Isono , Yuxi He , Mayuko Shimada , Kana Kato , Nan Jia , Satoru Hashimoto , Yuko Kotani , Yuka Miyoshi , Miyako Tanaka , Akira Sobue , Norisato Mitsutake , Takayoshi Suganami , Akio Masuda , Kinji Ohno , Shinichiro Nakada , Tomoji Mashimo , Koji Yamanaka , Martijn S. Luijsterburg , Tomoo Ogi
|
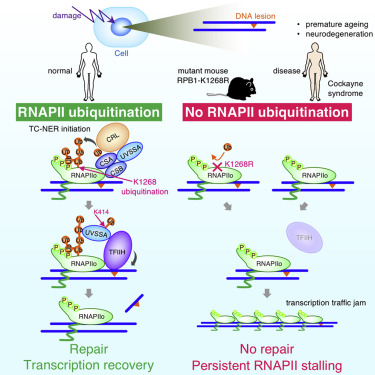
Transcription-coupled nucleotide excision repair (TC-NER) is initiated by the stalling of elongating RNA polymerase II (RNAPIIo) at DNA lesions. The ubiquitination of RNAPIIo in response to DNA damage is an evolutionarily conserved event, but its function in mammals is unknown. Here, we identified a single DNA damage-induced ubiquitination site in RNAPII at RPB1-K1268, which regulates transcription recovery and DNA damage resistance. Mechanistically, RPB1-K1268 ubiquitination stimulates the association of the core-TFIIH complex with stalled RNAPIIo through a transfer mechanism that also involves UVSSA-K414 ubiquitination. We developed a strand-specific ChIP-seq method, which revealed RPB1-K1268 ubiquitination is important for repair and the resolution of transcriptional bottlenecks at DNA lesions. Finally, RPB1-K1268R knockin mice displayed a short life-span, premature aging, and neurodegeneration. Our results reveal RNAPII ubiquitination provides a two-tier protection mechanism by activating TC-NER and, in parallel, the processing of DNA damage-stalled RNAPIIo, which together prevent prolonged transcription arrest and protect against neurodegeneration.
中文翻译:

DNA损伤停滞的RNAPII的泛素化促进转录偶联修复。
转录偶联核苷酸切除修复(TC-NER)是由DNA损伤处的延长RNA聚合酶II(RNAPIIo)停滞引起的。响应DNA损伤,RNAPIIo的泛素化是一个进化保守的事件,但其在哺乳动物中的功能尚不清楚。在这里,我们在RPB1-K1268的RNAPII中鉴定了单个DNA损伤诱导的泛素化位点,该位点调节转录恢复和DNA损伤抗性。从机理上讲,RPB1-K1268泛素化通过还涉及UVSSA-K414泛素化的转移机制刺激核心-TFIIH复合物与停滞的RNAPIIo缔合。我们开发了一种特定于链的ChIP-seq方法,该方法揭示了RPB1-K1268泛素化对于修复和解决DNA损伤处的转录瓶颈非常重要。最后,RPB1-K1268R敲入小鼠的寿命短,早衰和神经退行性变。我们的结果表明,RNAPII泛素化通过激活TC-NER并同时处理DNA损伤的RNAPIIo提供了两级保护机制,共同防止延长的转录停滞并保护神经变性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号