European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.ejmech.2020.112197 Liang Ma , Chen Yang , Jiaojiao Zheng , Yuchen Chen , Yushuo Xiao , Kun Huang
|
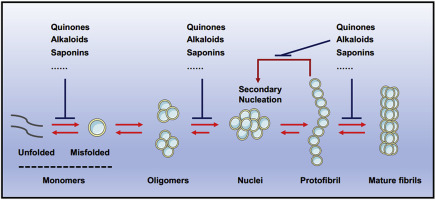
Protein misfolding diseases (PMDs) are chronic and progressive, with no effective therapy so far. Aggregation and misfolding of amyloidogenic proteins are closely associated with the onset and progression of PMDs, such as amyloid-β in Alzheimer's disease, α-Synuclein (α-Syn) in Parkinson's disease and human islet amyloid polypeptide (hIAPP) in type 2 diabetes. Inhibiting toxic aggregation of amyloidogenic proteins is regarded as a promising therapeutic approach in PMDs. The past decade has witnessed the rapid progresses of this field, dozens of inhibitors have been screened and verified in vitro and in vivo, demonstrating inhibitory effects against the aggregation and misfolding of amyloidogenic proteins, together with beneficial effects. Natural products are major sources of small molecule amyloid inhibitors, a number of natural derived compounds have been identified with great bioactivities and translational prospects. Here, we review the non-polyphenolic natural inhibitors that potentially applicable for PMDs treatment, along with their working mechanisms. Future directions are proposed for the development and clinical applications of these inhibitors.
中文翻译:

淀粉样蛋白聚集的非多酚天然抑制剂
蛋白质错误折叠病(PMD)是慢性和进行性疾病,目前尚无有效疗法。淀粉样蛋白原蛋白的聚集和错误折叠与PMD的发生和发展密切相关,例如阿尔茨海默氏病中的淀粉样蛋白β,帕金森氏病中的α-突触核蛋白(α-Syn)和2型糖尿病人胰岛淀粉样蛋白多肽(hIAPP)。抑制淀粉样蛋白的蛋白质的毒性聚集被认为是PMD中一种有前途的治疗方法。过去的十年见证了这一领域的快速进步,几十抑制剂已经被筛选和体外验证和在体内,证明了对淀粉样蛋白原蛋白的聚集和错误折叠的抑制作用以及有益作用。天然产物是小分子淀粉样蛋白抑制剂的主要来源,已鉴定出许多具有良好生物活性和翻译前景的天然衍生化合物。在这里,我们回顾了可能适用于PMD治疗的非多酚天然抑制剂及其工作机理。提出了这些抑制剂的开发和临床应用的未来方向。










































 京公网安备 11010802027423号
京公网安备 11010802027423号