Tetrahedron ( IF 2.1 ) Pub Date : 2020-03-05 , DOI: 10.1016/j.tet.2020.131088 Guillaume Bentzinger , Etienne Pair , Jean Guillon , Mathieu Marchivie , Catherine Mullié , Patrice Agnamey , Alexandra Dassonville-Klimpt , Pascal Sonnet
|
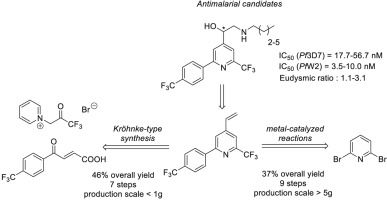
We describe the enantioselective synthesis and biological evaluation of 4-(2-amino-1-hydroxyethyl)pyridines (4 AHPs) as new antimalarial drug candidates. In particular, two routes to obtain the key-intermediate 4-vinyl-pyridine were studied. These routes are based on a Kröhnke-type cyclization or on metal-catalyzed reactions. The Kröhnke-type cyclization route is faster but only efficient at low scale since this pathway involves a Wittig reaction that requires severe temperature-control. Consequently, we designed a second route based on metal-catalyzed reactions. This way is longer but the 4-vinyl-pyridine can be obtained on a 5 g scale at least. Finally, a regioselective SN2 ring-opening of enantiopure epoxides by alkyl primary amines allowed the synthesis of eight 4-AHPs with global yields up to 41%. These compounds show strong in vitro antimalarial activity against P. falciparum strains and are more active that chloroquine and mefloquine. These results demonstrate that 4-AHPs are promising antimalarial drug candidates.
中文翻译:

对映体取代的吡啶是有前途的抗疟药物候选物
我们描述4-(2-氨基-1-羟乙基)吡啶(4 AHPs)作为新的抗疟药物候选物的对映选择性合成和生物学评估。特别地,研究了两种获得关键中间体4-乙烯基吡啶的途径。这些路线基于Kröhnke型环化反应或基于金属催化的反应。Kröhnke型环化途径较快,但仅在小规模生产时有效,因为该途径涉及需要严格控制温度的维蒂希反应。因此,我们设计了基于金属催化反应的第二条路线。这种方法更长,但是至少可以以5g的规模获得4-乙烯基吡啶。最后,区域选择性S N烷基伯胺对映体纯环氧化合物的2次开环允许合成8种4-AHP,总产率高达41%。这些化合物对恶性疟原虫菌株显示出强大的体外抗疟活性,并且比氯喹和甲氟喹更具活性。这些结果表明4-AHPs是有前途的抗疟药物候选物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号