当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adjustable Bioorthogonal Conjugation Platform for Protein Studies in Live Cells Based on Artificial Compartments.
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-03-17 , DOI: 10.1021/acssynbio.9b00494 Süreyya E Geissinger 1, 2 , Andreas Schreiber 1, 2 , Matthias C Huber 1, 2 , Lara G Stühn 1, 2 , Stefan M Schiller 1, 2, 3, 4, 5, 6
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-03-17 , DOI: 10.1021/acssynbio.9b00494 Süreyya E Geissinger 1, 2 , Andreas Schreiber 1, 2 , Matthias C Huber 1, 2 , Lara G Stühn 1, 2 , Stefan M Schiller 1, 2, 3, 4, 5, 6
Affiliation
|
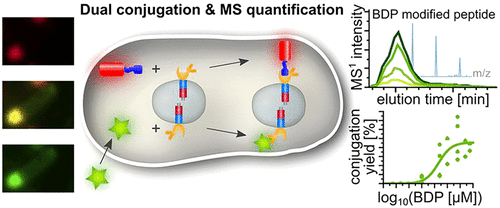
The investigation of complex biological processes in vivo often requires defined multiple bioconjugation and positioning of functional entities on 3D structures. Prominent examples include spatially defined protein complexes in nature, facilitating efficient biocatalysis of multistep reactions. Mimicking natural strategies, synthetic scaffolds should comprise bioorthogonal conjugation reactions and allow for absolute stoichiometric quantification as well as facile scalability through scaffold reproduction. Existing in vivo scaffolding strategies often lack covalent conjugations on geometrically confined scaffolds or precise quantitative characterization. Addressing these shortcomings, we present a bioorthogonal dual conjugation platform based on genetically encoded artificial compartments in vivo, comprising two distinct genetically encoded covalent conjugation reactions and their precise stoichiometric quantification. The SpyTag/SpyCatcher (ST/SC) bioconjugation and the controllable strain-promoted azide-alkyne cycloaddition (SPAAC) were implemented on self-assembled protein membrane-based compartments (PMBCs). The SPAAC reaction yield was quantified to be 23% ± 3% and a ST/SC surface conjugation yield of 82% ± 9% was observed, while verifying the compatibility of both chemical reactions as well as enhanced proteolytic stability. Using tandem mass spectrometry, absolute concentrations of the proteinaceous reactants were calculated to be 0.11 ± 0.05 attomol/cell for PMBC surface-tethered mCherry-ST-His and 0.22 ± 0.09 attomol/cell for PMBC-constituting pAzF-SC-E20F20-His. The established in vivo conjugation platform enables quantifiable protein-protein interaction studies on geometrically defined scaffolds and paves the road to investigate effects of scaffold-tethering on enzyme activity.
中文翻译:

可调节的生物正交偶联平台,用于基于人工隔室的活细胞蛋白质研究。
体内复杂生物过程的研究通常需要在3D结构上定义多个生物缀合和功能实体定位。突出的例子包括自然界中空间定义的蛋白质复合物,促进了多步反应的有效生物催化。模仿自然策略,合成支架应包括生物正交共轭反应,并允许绝对化学计量的定量以及通过支架复制的简便可扩展性。现有的体内支架策略通常缺乏在几何上受限的支架上的共价结合或精确的定量表征。针对这些缺点,我们提出了一种基于体内遗传编码的人工区隔的生物正交双重偶联平台,包含两个不同的遗传编码共价共轭反应及其精确的化学计量定量。SpyTag / SpyCatcher(ST / SC)生物缀合和可控应变促进的叠氮化物-炔烃环加成反应(SPAAC)在自组装蛋白膜隔室(PMBC)上实现。SPAAC反应收率定量为23%±3%,观察到的ST / SC表面结合收率为82%±9%,同时验证了两种化学反应的相容性以及增强的蛋白水解稳定性。使用串联质谱法,对于PMBC表面连接的mCherry-ST-His,蛋白质反应物的绝对浓度计算为0.11±0.05 attomol /细胞,对于PMBC构成的pAzF-SC-E20F20-His,其绝对浓度为0.22±0.09 attomol /细胞。
更新日期:2020-04-23
中文翻译:

可调节的生物正交偶联平台,用于基于人工隔室的活细胞蛋白质研究。
体内复杂生物过程的研究通常需要在3D结构上定义多个生物缀合和功能实体定位。突出的例子包括自然界中空间定义的蛋白质复合物,促进了多步反应的有效生物催化。模仿自然策略,合成支架应包括生物正交共轭反应,并允许绝对化学计量的定量以及通过支架复制的简便可扩展性。现有的体内支架策略通常缺乏在几何上受限的支架上的共价结合或精确的定量表征。针对这些缺点,我们提出了一种基于体内遗传编码的人工区隔的生物正交双重偶联平台,包含两个不同的遗传编码共价共轭反应及其精确的化学计量定量。SpyTag / SpyCatcher(ST / SC)生物缀合和可控应变促进的叠氮化物-炔烃环加成反应(SPAAC)在自组装蛋白膜隔室(PMBC)上实现。SPAAC反应收率定量为23%±3%,观察到的ST / SC表面结合收率为82%±9%,同时验证了两种化学反应的相容性以及增强的蛋白水解稳定性。使用串联质谱法,对于PMBC表面连接的mCherry-ST-His,蛋白质反应物的绝对浓度计算为0.11±0.05 attomol /细胞,对于PMBC构成的pAzF-SC-E20F20-His,其绝对浓度为0.22±0.09 attomol /细胞。











































 京公网安备 11010802027423号
京公网安备 11010802027423号