当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mapping of Ion and Substrate Binding Sites in Human Sodium Iodide Symporter (hNIS).
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-03-12 , DOI: 10.1021/acs.jcim.9b01114 Hristina R Zhekova 1 , Toshie Sakuma 2, 3 , Ryan Johnson 2 , Susanna C Concilio 3, 4 , Patrycja J Lech 2 , Igor Zdravkovic 1 , Mirna Damergi 1 , Lukkana Suksanpaisan 2 , Kah-Whye Peng 2, 3 , Stephen J Russell 2, 3 , Sergei Noskov 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-03-12 , DOI: 10.1021/acs.jcim.9b01114 Hristina R Zhekova 1 , Toshie Sakuma 2, 3 , Ryan Johnson 2 , Susanna C Concilio 3, 4 , Patrycja J Lech 2 , Igor Zdravkovic 1 , Mirna Damergi 1 , Lukkana Suksanpaisan 2 , Kah-Whye Peng 2, 3 , Stephen J Russell 2, 3 , Sergei Noskov 1
Affiliation
|
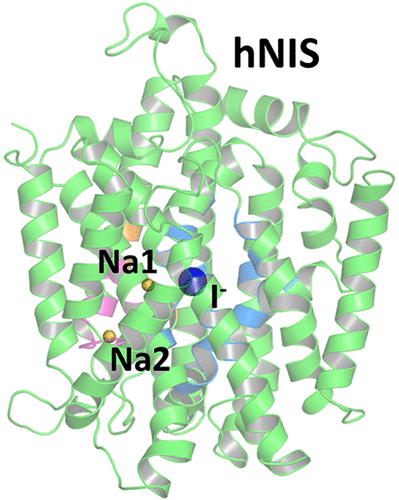
The human sodium iodide symporter (hNIS) is a theranostic reporter gene which concentrates several clinically approved SPECT and PET radiotracers and plays an essential role for the synthesis of thyroid hormones as an iodide transporter in the thyroid gland. Development of hNIS mutants which could enhance translocation of the desired imaging ions is currently underway. Unfortunately, it is hindered by lack of understanding of the 3D organization of hNIS and its relation to anion transport. There are no known crystal structures of hNIS in any of its conformational states. Homology modeling can be very effective in such situations; however, the low sequence identity between hNIS and relevant secondary transporters with available experimental structures makes the choice of a template and the generation of 3D models nontrivial. Here, we report a combined application of homology modeling and molecular dynamics refining of the hNIS structure in its semioccluded state. The modeling was based on templates from the LeuT-fold protein family and was done with emphasis on the refinement of the substrate-ion binding pocket. The consensus model developed in this work is compared to available biophysical and biochemical experimental data for a number of different LeuT-fold proteins. Some functionally important residues contributing to the formation of putative binding sites and permeation pathways for the cotransported Na+ ions and I- substrate were identified. The model predictions were experimentally tested by generation of mutant versions of hNIS and measurement of relative (to WT hNIS) 125I- uptake of 35 hNIS variants.
中文翻译:

人类碘化物钠转运蛋白(hNIS)中离子和底物结合位点的图谱。
人碘化钠同向转运蛋白(hNIS)是一种治疗诊断报告基因,其浓缩了几种临床认可的SPECT和PET放射性示踪剂,并且在甲状腺中作为碘化物转运体的合成甲状腺激素起着重要作用。目前正在开发可以增强所需成像离子易位的hNIS突变体。不幸的是,由于对hNIS的3D组织及其与阴离子迁移的关系缺乏了解而受到阻碍。没有任何处于其构象状态的hNIS晶体结构。同源建模在这种情况下可能非常有效。但是,hNIS与具有可用实验结构的相关二级转运蛋白之间的低序列同一性使模板的选择和3D模型的生成变得不平凡。这里,我们报道了在半封闭状态的hNIS结构的同源建模和分子动力学细化的组合应用。建模基于LeuT-fold蛋白质家族的模板,并着重于底物离子结合口袋的完善。将这项工作中开发的共有模型与许多不同LeuT折叠蛋白的可用生物物理和生化实验数据进行比较。确定了一些功能上重要的残基,这些残基有助于形成假定的结合位点和共转运的Na +离子和I-底物的渗透途径。通过生成hNIS的突变版本并测量35 hNIS变体的相对(相对于WT hNIS)125I吸收,对模型预测进行了实验测试。
更新日期:2020-04-24
中文翻译:

人类碘化物钠转运蛋白(hNIS)中离子和底物结合位点的图谱。
人碘化钠同向转运蛋白(hNIS)是一种治疗诊断报告基因,其浓缩了几种临床认可的SPECT和PET放射性示踪剂,并且在甲状腺中作为碘化物转运体的合成甲状腺激素起着重要作用。目前正在开发可以增强所需成像离子易位的hNIS突变体。不幸的是,由于对hNIS的3D组织及其与阴离子迁移的关系缺乏了解而受到阻碍。没有任何处于其构象状态的hNIS晶体结构。同源建模在这种情况下可能非常有效。但是,hNIS与具有可用实验结构的相关二级转运蛋白之间的低序列同一性使模板的选择和3D模型的生成变得不平凡。这里,我们报道了在半封闭状态的hNIS结构的同源建模和分子动力学细化的组合应用。建模基于LeuT-fold蛋白质家族的模板,并着重于底物离子结合口袋的完善。将这项工作中开发的共有模型与许多不同LeuT折叠蛋白的可用生物物理和生化实验数据进行比较。确定了一些功能上重要的残基,这些残基有助于形成假定的结合位点和共转运的Na +离子和I-底物的渗透途径。通过生成hNIS的突变版本并测量35 hNIS变体的相对(相对于WT hNIS)125I吸收,对模型预测进行了实验测试。











































 京公网安备 11010802027423号
京公网安备 11010802027423号