当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery and Development of Small-Molecule Inhibitors of Glycogen Synthase.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-03-23 , DOI: 10.1021/acs.jmedchem.9b01851 Buyun Tang 1 , Mykhaylo S Frasinyuk 2, 3 , Vimbai M Chikwana 1 , Krishna K Mahalingan 1 , Cynthia A Morgan 1 , Dyann M Segvich 1 , Svitlana P Bondarenko 3 , Galyna P Mrug 2, 3 , Przemyslaw Wyrebek 4, 5 , David S Watt 4, 5, 6 , Anna A DePaoli-Roach 1 , Peter J Roach 1 , Thomas D Hurley 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-03-23 , DOI: 10.1021/acs.jmedchem.9b01851 Buyun Tang 1 , Mykhaylo S Frasinyuk 2, 3 , Vimbai M Chikwana 1 , Krishna K Mahalingan 1 , Cynthia A Morgan 1 , Dyann M Segvich 1 , Svitlana P Bondarenko 3 , Galyna P Mrug 2, 3 , Przemyslaw Wyrebek 4, 5 , David S Watt 4, 5, 6 , Anna A DePaoli-Roach 1 , Peter J Roach 1 , Thomas D Hurley 1
Affiliation
|
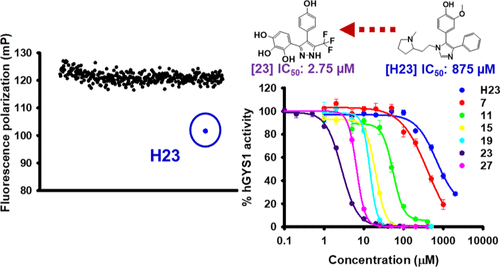
The overaccumulation of glycogen appears as a hallmark in various glycogen storage diseases (GSDs), including Pompe, Cori, Andersen, and Lafora disease. Accumulating evidence suggests that suppression of glycogen accumulation represents a potential therapeutic approach for treating these GSDs. Using a fluorescence polarization assay designed to screen for inhibitors of the key glycogen synthetic enzyme, glycogen synthase (GS), we identified a substituted imidazole, (rac)-2-methoxy-4-(1-(2-(1-methylpyrrolidin-2-yl)ethyl)-4-phenyl-1H-imidazol-5-yl)phenol (H23), as a first-in-class inhibitor for yeast GS 2 (yGsy2p). Data from X-ray crystallography at 2.85 Å, as well as kinetic data, revealed that H23 bound within the uridine diphosphate glucose binding pocket of yGsy2p. The high conservation of residues between human and yeast GS in direct contact with H23 informed the development of around 500 H23 analogs. These analogs produced a structure-activity relationship profile that led to the identification of a substituted pyrazole, 4-(4-(4-hydroxyphenyl)-3-(trifluoromethyl)-1H-pyrazol-5-yl)pyrogallol, with a 300-fold improved potency against human GS. These substituted pyrazoles possess a promising scaffold for drug development efforts targeting GS activity in GSDs associated with excess glycogen accumulation.
中文翻译:

糖原合酶的小分子抑制剂的发现和开发。
糖原的过度积累是各种糖原贮积病(GSD)的标志,包括庞贝病,科里病,安徒生病和拉福拉病。越来越多的证据表明,抑制糖原累积代表了治疗这些GSD的潜在治疗方法。使用设计用于筛选关键糖原合成酶,糖原合成酶(GS)抑制剂的荧光偏振分析,我们确定了取代的咪唑(rac)-2-甲氧基-4-(1-(2-(1-(1-甲基吡咯烷酮- (2-基)乙基)-4-苯基-1H-咪唑-5-基)苯酚(H23),是酵母GS 2(yGsy2p)的首批抑制剂。来自X射线晶体学在2.85Å处的数据以及动力学数据表明,H23结合在yGsy2p的尿苷二磷酸葡萄糖结合口袋中。直接与H23接触的人与酵母GS之间残基的高度保守性有助于开发约500种H23类似物。这些类似物产生了结构-活性关系图,从而鉴定了取代的吡唑4-(4-(4-羟苯基)-3-(三氟甲基)-1H-吡唑-5-基)邻苯三酚和300-对人GS的效力提高了两倍。这些取代的吡唑具有有前途的支架,可用于靶向与过量糖原积累相关的GSD中GS活性的药物开发工作。
更新日期:2020-03-24
中文翻译:

糖原合酶的小分子抑制剂的发现和开发。
糖原的过度积累是各种糖原贮积病(GSD)的标志,包括庞贝病,科里病,安徒生病和拉福拉病。越来越多的证据表明,抑制糖原累积代表了治疗这些GSD的潜在治疗方法。使用设计用于筛选关键糖原合成酶,糖原合成酶(GS)抑制剂的荧光偏振分析,我们确定了取代的咪唑(rac)-2-甲氧基-4-(1-(2-(1-(1-甲基吡咯烷酮- (2-基)乙基)-4-苯基-1H-咪唑-5-基)苯酚(H23),是酵母GS 2(yGsy2p)的首批抑制剂。来自X射线晶体学在2.85Å处的数据以及动力学数据表明,H23结合在yGsy2p的尿苷二磷酸葡萄糖结合口袋中。直接与H23接触的人与酵母GS之间残基的高度保守性有助于开发约500种H23类似物。这些类似物产生了结构-活性关系图,从而鉴定了取代的吡唑4-(4-(4-羟苯基)-3-(三氟甲基)-1H-吡唑-5-基)邻苯三酚和300-对人GS的效力提高了两倍。这些取代的吡唑具有有前途的支架,可用于靶向与过量糖原积累相关的GSD中GS活性的药物开发工作。











































 京公网安备 11010802027423号
京公网安备 11010802027423号