当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Robust De Novo-Designed Homotetrameric Coiled Coils
Biochemistry ( IF 2.9 ) Pub Date : 2020-03-09 , DOI: 10.1021/acs.biochem.0c00082 Caitlin L. Edgell 1, 2 , Nigel J. Savery 2, 3 , Derek N. Woolfson 1, 2, 3
Biochemistry ( IF 2.9 ) Pub Date : 2020-03-09 , DOI: 10.1021/acs.biochem.0c00082 Caitlin L. Edgell 1, 2 , Nigel J. Savery 2, 3 , Derek N. Woolfson 1, 2, 3
Affiliation
|
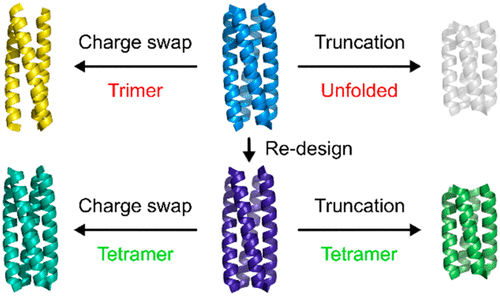
De novo-designed protein domains are increasingly being applied in biotechnology, cell biology, and synthetic biology. Therefore, it is imperative that these proteins be robust to superficial changes; i.e., small changes to their amino acid sequences should not cause gross structural changes. In turn, this allows properties such as stability and solubility to be tuned without affecting structural attributes like tertiary fold and quaternary interactions. Reliably designed proteins with predictable behaviors may then be used as scaffolds to incorporate function, e.g., through the introduction of features for small-molecule, metal, or macromolecular binding, and enzyme-like active sites. Generally, achieving this requires the starting protein fold to be well understood. Herein, we focus on designing α-helical coiled coils, which are well studied, widespread, and often direct protein–protein interactions in natural systems. Our initial investigations reveal that a previously designed parallel, homotetrameric coiled coil, CC-Tet, is not robust to sequence changes that were anticipated to maintain its structure. Instead, the alterations switch the oligomeric state from tetramer to trimer. To improve the robustness of designed homotetramers, additional sequences based on CC-Tet were produced and characterized in solution and by X-ray crystallography. Of these updated sequences, one is robust to truncation and to changes in surface electrostatics; we call this CC-Tet*. Variants of the general CC-Tet* design provide a set of homotetrameric coiled coils with unfolding temperatures in the range from 40 to >95 °C. We anticipate that these will be of use in applications requiring robust and well-defined tetramerization domains.
中文翻译:

耐用的De Novo-设计的同四聚体卷曲线圈
从头开始设计的蛋白质结构域正越来越多地应用于生物技术,细胞生物学和合成生物学。因此,这些蛋白质必须能抵抗表面变化。即,其氨基酸序列的微小变化不应引起总体结构变化。反过来,这允许在不影响诸如三级折叠和四级相互作用之类的结构属性的情况下调整诸如稳定性和溶解度之类的属性。然后,可以将具有可预测行为的可靠设计的蛋白质用作支架,以整合功能,例如通过引入小分子,金属或大分子结合特征以及酶样活性位点。通常,要实现这一点,就需要很好地理解起始蛋白质的折叠。在这里,我们专注于设计经过精心研究的α螺旋线圈,在自然系统中分布广泛且通常是直接的蛋白质间相互作用。我们的初步研究表明,先前设计的平行四聚体平行螺旋线圈CC-Tet对预期保持其结构的序列变化不稳健。相反,这些改变将寡聚状态从四聚体转变为三聚体。为了提高设计的同四聚体的稳健性,制备了基于CC-Tet的其他序列,并在溶液中和X射线晶体学对其进行了表征。在这些更新的序列中,一个对截断和表面静电变化具有鲁棒性。我们称之为CC-Tet *。常规CC-Tet *设计的变体提供了一组均四聚体卷曲螺旋,其展开温度范围为40至> 95°C。
更新日期:2020-03-09
中文翻译:

耐用的De Novo-设计的同四聚体卷曲线圈
从头开始设计的蛋白质结构域正越来越多地应用于生物技术,细胞生物学和合成生物学。因此,这些蛋白质必须能抵抗表面变化。即,其氨基酸序列的微小变化不应引起总体结构变化。反过来,这允许在不影响诸如三级折叠和四级相互作用之类的结构属性的情况下调整诸如稳定性和溶解度之类的属性。然后,可以将具有可预测行为的可靠设计的蛋白质用作支架,以整合功能,例如通过引入小分子,金属或大分子结合特征以及酶样活性位点。通常,要实现这一点,就需要很好地理解起始蛋白质的折叠。在这里,我们专注于设计经过精心研究的α螺旋线圈,在自然系统中分布广泛且通常是直接的蛋白质间相互作用。我们的初步研究表明,先前设计的平行四聚体平行螺旋线圈CC-Tet对预期保持其结构的序列变化不稳健。相反,这些改变将寡聚状态从四聚体转变为三聚体。为了提高设计的同四聚体的稳健性,制备了基于CC-Tet的其他序列,并在溶液中和X射线晶体学对其进行了表征。在这些更新的序列中,一个对截断和表面静电变化具有鲁棒性。我们称之为CC-Tet *。常规CC-Tet *设计的变体提供了一组均四聚体卷曲螺旋,其展开温度范围为40至> 95°C。











































 京公网安备 11010802027423号
京公网安备 11010802027423号