当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Congruent Release of Drug and Polymer from Amorphous Solid Dispersions: Insights into the Role of Drug-Polymer Hydrogen Bonding, Surface Crystallization, and Glass Transition.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-03-05 , DOI: 10.1021/acs.molpharmaceut.9b01272 Sugandha Saboo 1 , Umesh S Kestur 2 , Daniel P Flaherty 3 , Lynne S Taylor 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-03-05 , DOI: 10.1021/acs.molpharmaceut.9b01272 Sugandha Saboo 1 , Umesh S Kestur 2 , Daniel P Flaherty 3 , Lynne S Taylor 1
Affiliation
|
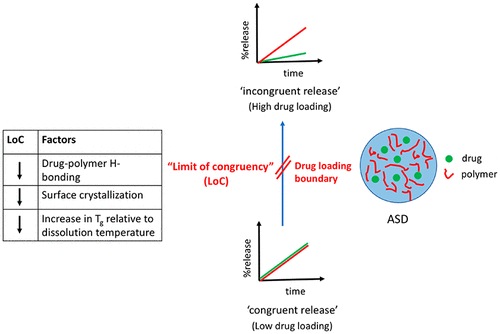
Drug loading is an important parameter known to impact the release rate of a poorly soluble drug from an amorphous solid dispersion (ASD). Recent studies have shown that small increases in drug loading can dramatically reduce the drug release rate from ASDs prepared with poly(vinylpyrrolidone-co-vinyl acetate) (PVPVA). However, the link between drug physicochemical properties and the drug loading where the release is abruptly compromised is not well understood. This study probes the role of different factors on the relative dissolution rates of drug and polymer from PVPVA-based ASDs as a function of drug loading: (1) the impact of drug-polymer hydrogen bonding interactions on the initial dissolution rate of ASDs, investigated using two structural analogues, indomethacin (IND) and indomethacin methyl ester (INDester), (2) the influence of surface drug crystallization, observed for INDester ASDs, and (3) by changing temperature, the impact of the “wet” glass transition temperature (Tg). Scanning electron microscopy (SEM), with or without energy dispersive X-ray (EDX) analysis, Fourier transform infrared spectroscopy (FTIR), and powder X-ray diffraction (PXRD) were utilized to study the solid-state phase behavior and/or drug enrichment on the partially dissolved ASD tablet surfaces. Nanoparticle tracking analysis (NTA) was utilized to study the solution-state phase behavior. It was found that, contrary to expectations, ASDs with drug-polymer hydrogen bonding exhibited poorer initial release at moderate drug loadings (15–25%) as compared to the non-hydrogen bonding analogue ASDs. Surface crystallization led to the deterioration of dissolution performance. Lastly, Tg relative to experimental temperatures also appeared to play a role in the observed dissolution behavior as a function of drug loading. These findings shed light on potential mechanisms governing ASD dissolution performance and will aid in the development of optimized ASD formulations with enhanced dissolution performance.
中文翻译:

非晶态固体分散体中药物和聚合物的同质释放:深入研究药物-聚合物氢键,表面结晶和玻璃化转变的作用。
载药量是已知的重要参数,会影响难溶性药物从无定形固体分散体(ASD)的释放速率。最近的研究表明,少量增加药量可以大大减少从聚准备自闭症药物的释放速度(vinylpyrrolidone-合作-乙酸乙烯酯(PVPVA)。但是,对于药物的物理化学性质和释放突然受到损害的载药量之间的关系还没有很好的了解。这项研究探讨了不同因素对基于PVPVA的ASDs药物和聚合物相对溶解速率与药物装载量的关系的作用:(1)药物-聚合物氢键相互作用对ASDs初始溶解速率的影响使用吲哚美辛(IND)和吲哚美辛甲酯(INDester)的两种结构类似物,(2)对于INDester ASD观察到的表面药物结晶的影响,以及(3)通过改变温度,“湿”玻璃化转变温度的影响(Ť克)。扫描电子显微镜(SEM),具有或不具有能量色散X射线(EDX)分析,傅里叶变换红外光谱(FTIR)和粉末X射线衍射(PXRD)均用于研究固态相行为和/或药物在部分溶解的ASD片剂表面上富集。纳米粒子跟踪分析(NTA)用于研究溶液状态的相行为。结果发现,与预期相反,具有药物-聚合物氢键的ASD与非氢键类似物ASD相比,在中等载药量(15-25%)下显示出较差的初始释放。表面结晶导致溶解性能下降。最后,T g相对于实验温度的相对值也似乎在观察到的溶出行为中与载药量有关。这些发现揭示了控制ASD溶解性能的潜在机制,并将有助于开发具有增强溶解性能的优化ASD配方。
更新日期:2020-04-24
中文翻译:

非晶态固体分散体中药物和聚合物的同质释放:深入研究药物-聚合物氢键,表面结晶和玻璃化转变的作用。
载药量是已知的重要参数,会影响难溶性药物从无定形固体分散体(ASD)的释放速率。最近的研究表明,少量增加药量可以大大减少从聚准备自闭症药物的释放速度(vinylpyrrolidone-合作-乙酸乙烯酯(PVPVA)。但是,对于药物的物理化学性质和释放突然受到损害的载药量之间的关系还没有很好的了解。这项研究探讨了不同因素对基于PVPVA的ASDs药物和聚合物相对溶解速率与药物装载量的关系的作用:(1)药物-聚合物氢键相互作用对ASDs初始溶解速率的影响使用吲哚美辛(IND)和吲哚美辛甲酯(INDester)的两种结构类似物,(2)对于INDester ASD观察到的表面药物结晶的影响,以及(3)通过改变温度,“湿”玻璃化转变温度的影响(Ť克)。扫描电子显微镜(SEM),具有或不具有能量色散X射线(EDX)分析,傅里叶变换红外光谱(FTIR)和粉末X射线衍射(PXRD)均用于研究固态相行为和/或药物在部分溶解的ASD片剂表面上富集。纳米粒子跟踪分析(NTA)用于研究溶液状态的相行为。结果发现,与预期相反,具有药物-聚合物氢键的ASD与非氢键类似物ASD相比,在中等载药量(15-25%)下显示出较差的初始释放。表面结晶导致溶解性能下降。最后,T g相对于实验温度的相对值也似乎在观察到的溶出行为中与载药量有关。这些发现揭示了控制ASD溶解性能的潜在机制,并将有助于开发具有增强溶解性能的优化ASD配方。











































 京公网安备 11010802027423号
京公网安备 11010802027423号