当前位置:
X-MOL 学术
›
JAMA Dermatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of Association Between Oral and Topical Terbinafine Use in Pregnancy and Risk of Major Malformations and Spontaneous Abortion.
JAMA Dermatology ( IF 11.5 ) Pub Date : 2020-04-01 , DOI: 10.1001/jamadermatol.2020.0142 Niklas Worm Andersson 1, 2 , Simon Francis Thomsen 3, 4 , Jon Trærup Andersen 1, 4
JAMA Dermatology ( IF 11.5 ) Pub Date : 2020-04-01 , DOI: 10.1001/jamadermatol.2020.0142 Niklas Worm Andersson 1, 2 , Simon Francis Thomsen 3, 4 , Jon Trærup Andersen 1, 4
Affiliation
|
Importance
Terbinafine is a commonly used antifungal agent, but safety data of its use in pregnancy are limited.
Objective
To examine the association between oral and topical terbinafine exposure in pregnancy and the risk of major malformations and spontaneous abortion.
Design, Setting, and Participants
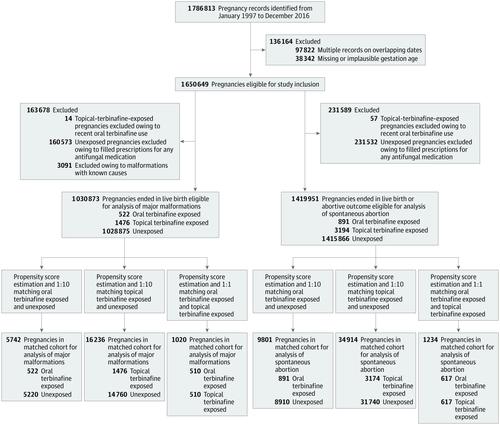
A nationwide, registry-based cohort study was conducted in Denmark from January 1, 1997, to December 31, 2016, in a cohort of 1 650 649 pregnancies. Data analysis was performed from July 11 to October 20, 2019. Pregnancies were matched on propensity scores comparing oral terbinafine exposed vs unexposed (1:10 ratio), topical terbinafine exposed vs unexposed (1:10), and oral vs topical terbinafine exposed (1:1).
Exposures
Filled prescriptions for oral or topical terbinafine.
Main Outcomes and Measures
Logistic regression was used to compute prevalence odds ratios for the primary outcome of major malformations and Cox proportional hazards regression was used to compute hazard ratios for the secondary outcome of spontaneous abortion.
Results
Based on a cohort of 1 650 649 pregnancies, oral terbinafine-exposed (n = 891 pregnancies; mean [SD] age, 30.4 [6] years) and topical terbinafine-exposed (n = 3174; mean [SD] age, 29.5 [5.4] years) pregnancies were identified; up to a total of 40 650 unexposed pregnancies were included for the matched outcome analyses. In propensity-matched comparisons of the risk of major malformations, the prevalence odds ratios were 1.01 (95% CI, 0.63-1.62) for oral terbinafine-exposed vs unexposed pregnancies (absolute risk difference [ARD], 0.04%; 95% CI, -1.69% to 1.76%), 1.08 (95% CI, 0.81-1.44) for topical terbinafine-exposed vs unexposed pregnancies (ARD, 0.26%; 95% CI, -0.73% to 1.26%), and 1.18 (95% CI, 0.61-2.29) for oral vs topical terbinafine-exposed pregnancies (ARD, 0.59%; 95% CI, -1.71% to 2.88%). For the risk of spontaneous abortion, the hazard ratios were 1.06 (95% CI, 0.86-1.32) for oral terbinafine-exposed vs unexposed pregnancies (ARD, 0.13%; 95% CI, -1.97% to 2.24%), 1.04 (95% CI, 0.88-1.21) for topical terbinafine-exposed vs unexposed pregnancies (ARD, 0.17%; 95% CI, -0.64% to 0.98%), and 1.19 (95% CI, 0.84-1.70) for oral vs topical terbinafine-exposed (ARD, 1.13%; 95% CI, -2.23% to 4.50%) pregnancies.
Conclusions and Relevance
Among pregnancies exposed to oral or topical terbinafine, no increased risk of major malformations or spontaneous abortion was identified.
中文翻译:

评价口服和局部使用特比萘芬在妊娠和严重畸形和自然流产风险中的关联。
重要性特比萘芬是一种常用的抗真菌剂,但在妊娠期使用它的安全性数据有限。目的探讨孕妇口服和局部特比萘芬暴露与严重畸形和自然流产风险之间的关系。设计,背景和参与者从1997年1月1日至2016年12月31日,在丹麦进行了一项基于注册表的全国性队列研究,队列1,650 649例。数据分析于2019年7月11日至10月20日进行。怀孕的倾向得分匹配,比较了口服特比萘芬暴露与未暴露(1:10的比例),局部特比萘芬暴露与未暴露(1:10的比例)以及口服特比萘芬暴露与局部暴露的特比萘芬(( 1:1)。暴露口服或局部特比萘芬的处方已满。主要结果和测量方法Logistic回归用于计算主要畸形主要结果的患病几率,Cox比例风险回归用于计算自然流产次要结果的风险比。结果基于1 650 649名孕妇的队列,口服特比萘芬暴露(n = 891;平均[SD]年龄为30.4 [6]岁)和局部使用特比萘芬暴露(n = 3174;平均[SD]年龄为29.5) [5.4年]怀孕被确定;多达40 650例未暴露的妊娠被纳入匹配的结果分析。在重大畸形风险的倾向匹配比较中,口服特比萘芬和未暴露孕妇的患病率比为1.01(95%CI,0.63-1.62)(绝对风险差异[ARD],0.04%; 95%CI, -1.69%至1.76%),1.08(95%CI,0。暴露于特比萘芬的局部和未暴露的孕妇(81.14.44)(ARD,0.26%; 95%CI,-0.73%至1.26%),以及口服与暴露于特比萘芬的局部与未暴露的怀孕(1.18(95%CI,0.61-2.29)( ARD:0.59%; 95%CI:-1.71%至2.88%)。对于自然流产的风险,口服特比萘芬暴露与未暴露孕妇的危险比分别为1.06(95%CI,0.86-1.32)(ARD,0.13%; 95%CI,-1.97%至2.24%),1.04(95特比萘芬局部用药和未特比萘芬局部用药的相对危险度(CI,0.88-1.21)(ARD,0.17%; 95%CI,-0.64%至0.98%)和口服特比萘芬-局部用药的1.19(95%CI,0.84-1.70)暴露(ARD,1.13%; 95%CI,-2.23%至4.50%)怀孕。结论和相关性在口服或局部特比萘芬暴露的孕妇中,未发现重大畸形或自然流产的风险增加。(44)暴露于特比萘芬的局部和未暴露的孕妇(ARD,0.26%; 95%CI,-0.73%至1.26%),口服与暴露于特比萘芬的局部与局部暴露的孕妇(ARD,1.72,95%CI,0.61-2.29) 0.59%; 95%CI,-1.71%至2.88%)。对于自然流产的风险,口服特比萘芬暴露与未暴露孕妇的危险比分别为1.06(95%CI,0.86-1.32)(ARD,0.13%; 95%CI,-1.97%至2.24%),1.04(95特比萘芬局部用药和未特比萘芬局部用药的相对危险度(CI,0.88-1.21)(ARD,0.17%; 95%CI,-0.64%至0.98%)和口服特比萘芬-局部用药的1.19(95%CI,0.84-1.70)暴露(ARD,1.13%; 95%CI,-2.23%至4.50%)怀孕。结论和相关性在口服或局部特比萘芬暴露的孕妇中,未发现重大畸形或自然流产的风险增加。(44)暴露于特比萘芬的局部和未暴露的孕妇(ARD,0.26%; 95%CI,-0.73%至1.26%),口服与暴露于特比萘芬的局部与局部暴露的孕妇(ARD,1.72,95%CI,0.61-2.29) 0.59%; 95%CI,-1.71%至2.88%)。对于自然流产的风险,口服特比萘芬暴露与未暴露孕妇的危险比分别为1.06(95%CI,0.86-1.32)(ARD,0.13%; 95%CI,-1.97%至2.24%),1.04(95特比萘芬局部用药与未用特比萘芬联合用药(%,CI)为0.88-1.21(ARD,0.17%; 95%CI,-0.64%至0.98%)和口服特比萘芬-局部用药为1.19(95%CI,0.84-1.70)暴露(ARD,1.13%; 95%CI,-2.23%至4.50%)怀孕。结论和相关性在口服或局部特比萘芬暴露的孕妇中,未发现重大畸形或自然流产的风险增加。口服和局部特比萘芬暴露的妊娠分别为1.18(95%CI,0.61-2.29)(ARD,0.59%; 95%CI,-1.71%至2.88%)。对于自然流产的风险,口服特比萘芬暴露与未暴露孕妇的危险比分别为1.06(95%CI,0.86-1.32)(ARD,0.13%; 95%CI,-1.97%至2.24%),1.04(95特比萘芬局部用药和未特比萘芬局部用药的相对危险度(CI,0.88-1.21)(ARD,0.17%; 95%CI,-0.64%至0.98%)和口服特比萘芬-局部用药的1.19(95%CI,0.84-1.70)暴露(ARD,1.13%; 95%CI,-2.23%至4.50%)怀孕。结论和相关性在口服或局部特比萘芬暴露的孕妇中,未发现重大畸形或自然流产的风险增加。口服和局部特比萘芬暴露的妊娠分别为1.18(95%CI,0.61-2.29)(ARD,0.59%; 95%CI,-1.71%至2.88%)。对于自然流产的风险,口服特比萘芬暴露与未暴露孕妇的危险比分别为1.06(95%CI,0.86-1.32)(ARD,0.13%; 95%CI,-1.97%至2.24%),1.04(95特比萘芬局部用药和未特比萘芬局部用药的相对危险度(CI,0.88-1.21)(ARD,0.17%; 95%CI,-0.64%至0.98%)和口服特比萘芬-局部用药的1.19(95%CI,0.84-1.70)暴露(ARD,1.13%; 95%CI,-2.23%至4.50%)怀孕。结论和相关性在口服或局部特比萘芬暴露的孕妇中,未发现重大畸形或自然流产的风险增加。口服特比萘芬与未暴露孕妇的危险比分别为1.06(95%CI,0.86-1.32)(ARD,0.13%; 95%CI,-1.97%至2.24%),1.04(95%CI,0.88-1.21)暴露于特比萘芬的局部和未暴露的孕妇(ARD,0.17%; 95%CI,-0.64%至0.98%),口服vs暴露于特比萘芬的局部妊娠(ARD,1.13%; 95%CI,0.86-1.70) 95%CI,-2.23%至4.50%)怀孕。结论和相关性在口服或局部特比萘芬暴露的孕妇中,未发现重大畸形或自然流产的风险增加。口服特比萘芬与未暴露孕妇的危险比分别为1.06(95%CI,0.86-1.32)(ARD,0.13%; 95%CI,-1.97%至2.24%),1.04(95%CI,0.88-1.21)暴露于特比萘芬的局部和未暴露的孕妇(ARD,0.17%; 95%CI,-0.64%至0.98%),口服和暴露于特比萘芬的局部与未暴露的怀孕(ARD,1.13%; 1.91%; 95%CI,0.84-1.70)。 95%CI,-2.23%至4.50%)怀孕。结论与相关性在暴露于口服或局部特比萘芬的妊娠中,未发现重大畸形或自然流产的风险增加。70)口服和局部暴露于特比萘芬的妊娠(ARD,1.13%; 95%CI,-2.23%至4.50%)。结论和相关性在口服或局部特比萘芬暴露的孕妇中,未发现重大畸形或自然流产的风险增加。70)口服和局部暴露于特比萘芬的妊娠(ARD,1.13%; 95%CI,-2.23%至4.50%)。结论和相关性在口服或局部特比萘芬暴露的孕妇中,未发现重大畸形或自然流产的风险增加。
更新日期:2020-04-01
中文翻译:

评价口服和局部使用特比萘芬在妊娠和严重畸形和自然流产风险中的关联。
重要性特比萘芬是一种常用的抗真菌剂,但在妊娠期使用它的安全性数据有限。目的探讨孕妇口服和局部特比萘芬暴露与严重畸形和自然流产风险之间的关系。设计,背景和参与者从1997年1月1日至2016年12月31日,在丹麦进行了一项基于注册表的全国性队列研究,队列1,650 649例。数据分析于2019年7月11日至10月20日进行。怀孕的倾向得分匹配,比较了口服特比萘芬暴露与未暴露(1:10的比例),局部特比萘芬暴露与未暴露(1:10的比例)以及口服特比萘芬暴露与局部暴露的特比萘芬(( 1:1)。暴露口服或局部特比萘芬的处方已满。主要结果和测量方法Logistic回归用于计算主要畸形主要结果的患病几率,Cox比例风险回归用于计算自然流产次要结果的风险比。结果基于1 650 649名孕妇的队列,口服特比萘芬暴露(n = 891;平均[SD]年龄为30.4 [6]岁)和局部使用特比萘芬暴露(n = 3174;平均[SD]年龄为29.5) [5.4年]怀孕被确定;多达40 650例未暴露的妊娠被纳入匹配的结果分析。在重大畸形风险的倾向匹配比较中,口服特比萘芬和未暴露孕妇的患病率比为1.01(95%CI,0.63-1.62)(绝对风险差异[ARD],0.04%; 95%CI, -1.69%至1.76%),1.08(95%CI,0。暴露于特比萘芬的局部和未暴露的孕妇(81.14.44)(ARD,0.26%; 95%CI,-0.73%至1.26%),以及口服与暴露于特比萘芬的局部与未暴露的怀孕(1.18(95%CI,0.61-2.29)( ARD:0.59%; 95%CI:-1.71%至2.88%)。对于自然流产的风险,口服特比萘芬暴露与未暴露孕妇的危险比分别为1.06(95%CI,0.86-1.32)(ARD,0.13%; 95%CI,-1.97%至2.24%),1.04(95特比萘芬局部用药和未特比萘芬局部用药的相对危险度(CI,0.88-1.21)(ARD,0.17%; 95%CI,-0.64%至0.98%)和口服特比萘芬-局部用药的1.19(95%CI,0.84-1.70)暴露(ARD,1.13%; 95%CI,-2.23%至4.50%)怀孕。结论和相关性在口服或局部特比萘芬暴露的孕妇中,未发现重大畸形或自然流产的风险增加。(44)暴露于特比萘芬的局部和未暴露的孕妇(ARD,0.26%; 95%CI,-0.73%至1.26%),口服与暴露于特比萘芬的局部与局部暴露的孕妇(ARD,1.72,95%CI,0.61-2.29) 0.59%; 95%CI,-1.71%至2.88%)。对于自然流产的风险,口服特比萘芬暴露与未暴露孕妇的危险比分别为1.06(95%CI,0.86-1.32)(ARD,0.13%; 95%CI,-1.97%至2.24%),1.04(95特比萘芬局部用药和未特比萘芬局部用药的相对危险度(CI,0.88-1.21)(ARD,0.17%; 95%CI,-0.64%至0.98%)和口服特比萘芬-局部用药的1.19(95%CI,0.84-1.70)暴露(ARD,1.13%; 95%CI,-2.23%至4.50%)怀孕。结论和相关性在口服或局部特比萘芬暴露的孕妇中,未发现重大畸形或自然流产的风险增加。(44)暴露于特比萘芬的局部和未暴露的孕妇(ARD,0.26%; 95%CI,-0.73%至1.26%),口服与暴露于特比萘芬的局部与局部暴露的孕妇(ARD,1.72,95%CI,0.61-2.29) 0.59%; 95%CI,-1.71%至2.88%)。对于自然流产的风险,口服特比萘芬暴露与未暴露孕妇的危险比分别为1.06(95%CI,0.86-1.32)(ARD,0.13%; 95%CI,-1.97%至2.24%),1.04(95特比萘芬局部用药与未用特比萘芬联合用药(%,CI)为0.88-1.21(ARD,0.17%; 95%CI,-0.64%至0.98%)和口服特比萘芬-局部用药为1.19(95%CI,0.84-1.70)暴露(ARD,1.13%; 95%CI,-2.23%至4.50%)怀孕。结论和相关性在口服或局部特比萘芬暴露的孕妇中,未发现重大畸形或自然流产的风险增加。口服和局部特比萘芬暴露的妊娠分别为1.18(95%CI,0.61-2.29)(ARD,0.59%; 95%CI,-1.71%至2.88%)。对于自然流产的风险,口服特比萘芬暴露与未暴露孕妇的危险比分别为1.06(95%CI,0.86-1.32)(ARD,0.13%; 95%CI,-1.97%至2.24%),1.04(95特比萘芬局部用药和未特比萘芬局部用药的相对危险度(CI,0.88-1.21)(ARD,0.17%; 95%CI,-0.64%至0.98%)和口服特比萘芬-局部用药的1.19(95%CI,0.84-1.70)暴露(ARD,1.13%; 95%CI,-2.23%至4.50%)怀孕。结论和相关性在口服或局部特比萘芬暴露的孕妇中,未发现重大畸形或自然流产的风险增加。口服和局部特比萘芬暴露的妊娠分别为1.18(95%CI,0.61-2.29)(ARD,0.59%; 95%CI,-1.71%至2.88%)。对于自然流产的风险,口服特比萘芬暴露与未暴露孕妇的危险比分别为1.06(95%CI,0.86-1.32)(ARD,0.13%; 95%CI,-1.97%至2.24%),1.04(95特比萘芬局部用药和未特比萘芬局部用药的相对危险度(CI,0.88-1.21)(ARD,0.17%; 95%CI,-0.64%至0.98%)和口服特比萘芬-局部用药的1.19(95%CI,0.84-1.70)暴露(ARD,1.13%; 95%CI,-2.23%至4.50%)怀孕。结论和相关性在口服或局部特比萘芬暴露的孕妇中,未发现重大畸形或自然流产的风险增加。口服特比萘芬与未暴露孕妇的危险比分别为1.06(95%CI,0.86-1.32)(ARD,0.13%; 95%CI,-1.97%至2.24%),1.04(95%CI,0.88-1.21)暴露于特比萘芬的局部和未暴露的孕妇(ARD,0.17%; 95%CI,-0.64%至0.98%),口服vs暴露于特比萘芬的局部妊娠(ARD,1.13%; 95%CI,0.86-1.70) 95%CI,-2.23%至4.50%)怀孕。结论和相关性在口服或局部特比萘芬暴露的孕妇中,未发现重大畸形或自然流产的风险增加。口服特比萘芬与未暴露孕妇的危险比分别为1.06(95%CI,0.86-1.32)(ARD,0.13%; 95%CI,-1.97%至2.24%),1.04(95%CI,0.88-1.21)暴露于特比萘芬的局部和未暴露的孕妇(ARD,0.17%; 95%CI,-0.64%至0.98%),口服和暴露于特比萘芬的局部与未暴露的怀孕(ARD,1.13%; 1.91%; 95%CI,0.84-1.70)。 95%CI,-2.23%至4.50%)怀孕。结论与相关性在暴露于口服或局部特比萘芬的妊娠中,未发现重大畸形或自然流产的风险增加。70)口服和局部暴露于特比萘芬的妊娠(ARD,1.13%; 95%CI,-2.23%至4.50%)。结论和相关性在口服或局部特比萘芬暴露的孕妇中,未发现重大畸形或自然流产的风险增加。70)口服和局部暴露于特比萘芬的妊娠(ARD,1.13%; 95%CI,-2.23%至4.50%)。结论和相关性在口服或局部特比萘芬暴露的孕妇中,未发现重大畸形或自然流产的风险增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号