当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Target Validation of Aspartate Transcarbamoylase from Plasmodium falciparum by Torin 2.
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2020-03-12 , DOI: 10.1021/acsinfecdis.9b00411 Soraya S Bosch 1, 2 , Sergey Lunev 2 , Fernando A Batista 2 , Marleen Linzke 1 , Thales Kronenberger 3 , Alexander S S Dömling 2 , Matthew R Groves 2 , Carsten Wrenger 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2020-03-12 , DOI: 10.1021/acsinfecdis.9b00411 Soraya S Bosch 1, 2 , Sergey Lunev 2 , Fernando A Batista 2 , Marleen Linzke 1 , Thales Kronenberger 3 , Alexander S S Dömling 2 , Matthew R Groves 2 , Carsten Wrenger 1
Affiliation
|
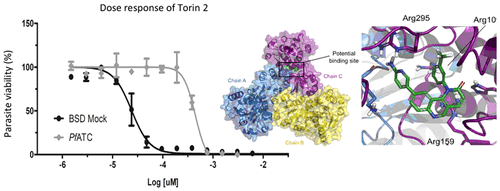
Malaria is a tropical disease that kills about half a million people around the world annually. Enzymatic reactions within pyrimidine biosynthesis have been proven to be essential for Plasmodium proliferation. Here we report on the essentiality of the second enzymatic step of the pyrimidine biosynthesis pathway, catalyzed by aspartate transcarbamoylase (ATC). Crystallization experiments using a double mutant ofPlasmodium falciparum ATC (PfATC) revealed the importance of the mutated residues for enzyme catalysis. Subsequently, this mutant was employed in protein interference assays (PIAs), which resulted in inhibition of parasite proliferation when parasites transfected with the double mutant were cultivated in medium lacking an excess of nutrients, including aspartate. Addition of 5 or 10 mg/L of aspartate to the minimal medium restored the parasites' normal growth rate. In vitro and whole-cell assays in the presence of the compound Torin 2 showed inhibition of specific activity and parasite growth, respectively. In silico analyses revealed the potential binding mode of Torin 2 to PfATC. Furthermore, a transgenic ATC-overexpressing cell line exhibited a 10-fold increased tolerance to Torin 2 compared with control cultures. Taken together, our results confirm the antimalarial activity of Torin 2, suggesting PfATC as a target of this drug and a promising target for the development of novel antimalarials.
中文翻译:

都灵2从恶性疟原虫中天冬氨酸转氨甲酰酶的分子靶标验证。
疟疾是一种热带疾病,每年全世界约有一百万人丧生。嘧啶生物合成中的酶促反应已被证明对疟原虫增殖至关重要。在这里我们报告了由天冬氨酸转氨甲酰酶(ATC)催化的嘧啶生物合成途径的第二个酶促步骤的必要性。使用恶性疟原虫ATC(PfATC)的双重突变体的结晶实验揭示了突变残基对酶催化的重要性。随后,将该突变体用于蛋白质干扰检测(PIA),当将用双突变体转染的寄生虫培养在缺乏营养的培养基(包括天冬氨酸)中时,可抑制寄生虫的增殖。在基本培养基中添加5或10 mg / L的天冬氨酸可恢复寄生虫的正常生长速度。在存在化合物Torin 2的情况下的体外和全细胞分析分别显示了对特定活性和寄生虫生长的抑制。在计算机分析中揭示了都灵2与PfATC的潜在结合模式。此外,与对照培养物相比,转基因的过表达ATC的细胞系对Torin 2的耐受性提高了10倍。综上所述,我们的结果证实了Torin 2的抗疟活性,表明PfATC是该药物的靶标,也是开发新型抗疟药的有希望的靶标。在计算机分析中揭示了都灵2与PfATC的潜在结合模式。此外,与对照培养物相比,转基因的过表达ATC的细胞系对Torin 2的耐受性提高了10倍。综上所述,我们的结果证实了Torin 2的抗疟活性,表明PfATC是该药物的靶标,也是开发新型抗疟药的有希望的靶标。在计算机分析中揭示了都灵2与PfATC的潜在结合模式。此外,与对照培养物相比,转基因的过表达ATC的细胞系对Torin 2的耐受性提高了10倍。综上所述,我们的结果证实了Torin 2的抗疟活性,表明PfATC是该药物的靶标,也是开发新型抗疟药的有希望的靶标。
更新日期:2020-03-04
中文翻译:

都灵2从恶性疟原虫中天冬氨酸转氨甲酰酶的分子靶标验证。
疟疾是一种热带疾病,每年全世界约有一百万人丧生。嘧啶生物合成中的酶促反应已被证明对疟原虫增殖至关重要。在这里我们报告了由天冬氨酸转氨甲酰酶(ATC)催化的嘧啶生物合成途径的第二个酶促步骤的必要性。使用恶性疟原虫ATC(PfATC)的双重突变体的结晶实验揭示了突变残基对酶催化的重要性。随后,将该突变体用于蛋白质干扰检测(PIA),当将用双突变体转染的寄生虫培养在缺乏营养的培养基(包括天冬氨酸)中时,可抑制寄生虫的增殖。在基本培养基中添加5或10 mg / L的天冬氨酸可恢复寄生虫的正常生长速度。在存在化合物Torin 2的情况下的体外和全细胞分析分别显示了对特定活性和寄生虫生长的抑制。在计算机分析中揭示了都灵2与PfATC的潜在结合模式。此外,与对照培养物相比,转基因的过表达ATC的细胞系对Torin 2的耐受性提高了10倍。综上所述,我们的结果证实了Torin 2的抗疟活性,表明PfATC是该药物的靶标,也是开发新型抗疟药的有希望的靶标。在计算机分析中揭示了都灵2与PfATC的潜在结合模式。此外,与对照培养物相比,转基因的过表达ATC的细胞系对Torin 2的耐受性提高了10倍。综上所述,我们的结果证实了Torin 2的抗疟活性,表明PfATC是该药物的靶标,也是开发新型抗疟药的有希望的靶标。在计算机分析中揭示了都灵2与PfATC的潜在结合模式。此外,与对照培养物相比,转基因的过表达ATC的细胞系对Torin 2的耐受性提高了10倍。综上所述,我们的结果证实了Torin 2的抗疟活性,表明PfATC是该药物的靶标,也是开发新型抗疟药的有希望的靶标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号