Molecular and Biochemical Parasitology ( IF 1.5 ) Pub Date : 2020-02-11 , DOI: 10.1016/j.molbiopara.2020.111266 Ishita Gupta 1 , Sameena Khan 2
|
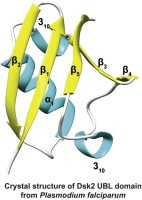
One of the pathways by which proteins are targeted for degradation by the proteasome involve transport by shuttle proteins to proteasomal receptors. The malaria parasite Plasmodium falciparum has recently been found to possess a similar pathway, with the shuttle protein PfDsk2 being the major player. In this study, we have demonstrated how PfDsk2 and its recognition by proteasomal receptors differ from the mammalian system. Our crystal structure of unbound PfDsk2 UBL domain at 1.30 Å revealed an additional 310-helix compared to the human homolog, as well as a few significant differences in its putative binding interface with the proteasome receptors, PfRpn10 and PfRpn13. Moreover, the non-binding face of UBL showed a reversal of surface charge compared to HsDsk2 shuttle protein, instead resembling HOIL-like E3 ligase UBL domain. The affinity of the interaction with the proteasomal receptors remained similar to the human system, and dissociation constants of the same order of magnitude. On the other hand, we have found evidence of a novel interaction between PfRpn13DEUBAD with the PfDsk2UBL suggesting that PfDsk2 may work in cooperation with deubiquitinating enzymes for proofreading ubiquitinated substrates. Our study provides the first molecular look at shuttle proteins in Apicomplexan parasites and hints at how their interaction landscape might be broader than what we may expect.
中文翻译:

恶性疟原虫DSK2对蛋白酶体受体的识别。
蛋白质被蛋白酶体靶向降解的途径之一涉及通过穿梭蛋白转运至蛋白酶体受体。最近发现疟疾寄生虫恶性疟原虫具有类似的途径,其中穿梭蛋白PfDsk2是主要参与者。在这项研究中,我们已经证明了PfDsk2及其蛋白酶体受体的识别与哺乳动物系统有何不同。我们在1.30Å处未结合的PfDsk2 UBL结构域的晶体结构揭示了另外3 10-螺旋与人类同系物相比,以及其与蛋白酶体受体PfRpn10和PfRpn13的推定结合界面有一些显着差异。此外,与HsDsk2穿梭蛋白相比,UBL的非结合面显示出表面电荷的反转,而不像HOIL样E3连接酶UBL结构域。与蛋白酶体受体相互作用的亲和力仍然与人类系统相似,并且解离常数处于相同数量级。另一方面,我们发现了PfRpn13 DEUBAD与PfDsk2 UBL之间发生新型相互作用的证据提示PfDsk2可能与去泛素化酶协同作用以校对泛素化底物。我们的研究首次提供了对Apicomplexan寄生虫中穿梭蛋白的分子研究,并暗示了它们的相互作用前景可能比我们预期的更广泛。



























 京公网安备 11010802027423号
京公网安备 11010802027423号