Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2020-01-31 , DOI: 10.1016/j.csbj.2020.01.008 Kathleen Sprouffske 1 , Grainne Kerr 1 , Cheng Li 1 , Anirudh Prahallad 1 , Ramona Rebmann 1 , Verena Waehle 1 , Ulrike Naumann 2 , Hans Bitter 3 , Michael R Jensen 1 , Francesco Hofmann 1 , Saskia M Brachmann 1 , Stéphane Ferretti 1 , Audrey Kauffmann 1
|
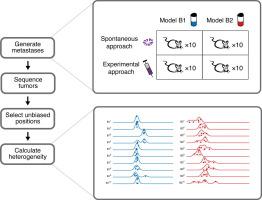
Genetic heterogeneity within a tumor arises by clonal evolution, and patients with highly heterogeneous tumors are more likely to be resistant to therapy and have reduced survival. Clonal evolution also occurs when a subset of cells leave the primary tumor to form metastases, which leads to reduced genetic heterogeneity at the metastatic site. Although this process has been observed in human cancer, experimental models which recapitulate this process are lacking. Patient-derived tumor xenografts (PDX) have been shown to recapitulate the patient’s original tumor’s intra-tumor genetic heterogeneity, as well as its genomics and response to treatment, but whether they can be used to model clonal evolution in the metastatic process is currently unknown. Here, we address this question by following genetic changes in two breast cancer PDX models during metastasis. First, we discovered that mouse stroma can be a confounding factor in assessing intra-tumor heterogeneity by whole exome sequencing, thus we developed a new bioinformatic approach to correct for this. Finally, in a spontaneous, but not experimental (tail-vein) metastasis model we observed a loss of heterogeneity in PDX metastases compared to their orthotopic “primary” tumors, confirming that PDX models can faithfully mimic the clonal evolution process undergone in human patients during metastatic spreading.
中文翻译:

乳腺癌患者源性肿瘤异种移植模型转移过程中的遗传异质性和克隆进化。
肿瘤内的遗传异质性是由克隆进化引起的,具有高度异质性肿瘤的患者更有可能对治疗产生耐药性并降低生存率。当一部分细胞离开原发性肿瘤形成转移灶时,也会发生克隆进化,从而导致转移部位的遗传异质性降低。尽管在人类癌症中已经观察到该过程,但是缺乏概括该过程的实验模型。已显示患者源性肿瘤异种移植物(PDX)可概括患者原始肿瘤的肿瘤内遗传异质性及其基因组学和对治疗的反应,但目前尚不清楚它们是否可用于模拟转移过程中的克隆进化。这里,我们通过追踪两个乳腺癌PDX模型在转移过程中的遗传变化来解决这个问题。首先,我们发现小鼠基质可能是通过整个外显子组测序评估肿瘤内异质性的一个混杂因素,因此我们开发了一种新的生物信息学方法来对此进行纠正。最后,在自发但非实验性(尾静脉)转移模型中,我们观察到与原位“原发性”肿瘤相比,PDX转移的异质性丧失,这证实了PDX模型可以忠实地模拟人类患者在此期间的克隆进化过程。转移性扩散。











































 京公网安备 11010802027423号
京公网安备 11010802027423号