当前位置:
X-MOL 学术
›
J. Struct. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Remote oxidative modifications induced by oxygen free radicals modify T/R allosteric equilibrium of a hyperthermophilic lactate dehydrogenase.
Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.jsb.2020.107478 Frédéric Halgand 1 , Chantal Houée-Lévin 1 , Martin Weik 2 , Dominique Madern 2
Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.jsb.2020.107478 Frédéric Halgand 1 , Chantal Houée-Lévin 1 , Martin Weik 2 , Dominique Madern 2
Affiliation
|
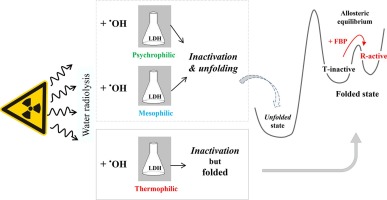
L-Lactate dehydrogenase (LDH) is a model protein allowing to shed light on the fundamental molecular mechanisms that drive the acquisition, evolution and regulation of enzyme properties. In this study, we test the hypothesis of a link between thermal stability of LDHs and their capacity against unfolding induced by reactive oxygen species (ROS) generated by γ-rays irradiation. By using circular dichroism spectroscopy, we analysed that high thermal stability of a thermophilic LDH favours strong resistance against ROS-induced unfolding, in contrast to its psychrophilic and mesophilic counterparts that are less resistant. We suggest that a protein's phenotype linking strong thermal stability and resistance against ROS damages would have been a selective evolutionary advantage. We also find that the enzymatic activity of the thermophilic LDH that is strongly resistant against ROS-unfolding is very sensitive to inactivation by irradiation. To address this counter-intuitive observation, we combined mass spectrometry analyses and enzymatic activity measurements. We demonstrate that the dramatic change on LDH activity was linked to remote chemical modifications away from the active site, that change the equilibrium between low-affinity tense (T-inactive) and high-affinity relaxed (R-active) forms. We found the T-inactive thermophilic enzyme obtained after irradiation can recover its LDH activity by addition of the allosteric effector 1, 6 fructose bis phosphate. We analyse our data within the general framework of allosteric regulation, which requires that an enzyme in solution populates a large diversity of dynamically-interchanging conformations. Our work demonstrates that the radiation-induced inactivation of an enzyme is controlled by its dynamical properties.
中文翻译:

由氧自由基诱导的远程氧化修饰修饰了超嗜热乳酸脱氢酶的T / R变构平衡。
L-乳酸脱氢酶(LDH)是一种模型蛋白,可以阐明驱动酶特性获取,进化和调节的基本分子机制。在这项研究中,我们测试了LDH的热稳定性与其抵抗由γ射线辐射产生的活性氧(ROS)诱导的解折叠能力之间联系的假设。通过使用圆二色性光谱,我们分析了嗜热LDH的高热稳定性有利于抵抗ROS诱导的展开,而耐低温和嗜温的对应物则较弱。我们认为,连接强大的热稳定性和对ROS损害的抵抗力的蛋白质表型将是选择性的进化优势。我们还发现,强烈抵抗ROS折叠的嗜热LDH的酶促活性对辐射灭活非常敏感。为了解决这种违反直觉的观察,我们将质谱分析和酶活性测量相结合。我们证明,LDH活性的戏剧性变化与远离活性位点的远程化学修饰有关,从而改变了低亲和力时态(T-非活性)和高亲和力时态(R-活性)形式之间的平衡。我们发现,辐照后获得的T非活性嗜热酶可以通过添加变构效应物1、6果糖双磷酸酯来恢复其LDH活性。我们在变构调节的一般框架内分析数据,这就要求溶液中的酶能够组成各种各样的动态互换构象。我们的工作表明,辐射诱导的酶失活受其动力学特性控制。
更新日期:2020-03-26
中文翻译:

由氧自由基诱导的远程氧化修饰修饰了超嗜热乳酸脱氢酶的T / R变构平衡。
L-乳酸脱氢酶(LDH)是一种模型蛋白,可以阐明驱动酶特性获取,进化和调节的基本分子机制。在这项研究中,我们测试了LDH的热稳定性与其抵抗由γ射线辐射产生的活性氧(ROS)诱导的解折叠能力之间联系的假设。通过使用圆二色性光谱,我们分析了嗜热LDH的高热稳定性有利于抵抗ROS诱导的展开,而耐低温和嗜温的对应物则较弱。我们认为,连接强大的热稳定性和对ROS损害的抵抗力的蛋白质表型将是选择性的进化优势。我们还发现,强烈抵抗ROS折叠的嗜热LDH的酶促活性对辐射灭活非常敏感。为了解决这种违反直觉的观察,我们将质谱分析和酶活性测量相结合。我们证明,LDH活性的戏剧性变化与远离活性位点的远程化学修饰有关,从而改变了低亲和力时态(T-非活性)和高亲和力时态(R-活性)形式之间的平衡。我们发现,辐照后获得的T非活性嗜热酶可以通过添加变构效应物1、6果糖双磷酸酯来恢复其LDH活性。我们在变构调节的一般框架内分析数据,这就要求溶液中的酶能够组成各种各样的动态互换构象。我们的工作表明,辐射诱导的酶失活受其动力学特性控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号