Current Organic Synthesis ( IF 1.7 ) Pub Date : 2020-05-01 , DOI: 10.2174/1570179417666200226092516 El-Sayed M Abdelrehim 1 , Doaa S El-Sayed 2
|
Background: 2-amino-3-cyanopyridines are good starting reagents that have been used in synthesis of many heterocyclic compounds such as pyridopyrimidines, [1,2,4]triazolo and [1,2,3,4] tetrazolo derivatives which have biological activities as anti-microbial and cytotoxic activities. Meanwhile [1,2,4]triazolo and [1,2,3,4]tetrazolo derivatives are well known to possess many physiological activities, such as anticancer , antifungal, muscle relaxant, hypnotic, anti-inflammatory, diuretic and antihypertensive activities. A broad class of heterocyclic compounds has been studied to demonstrate their biological activity on the structures of DNA and RNA. Several of important functions make Tankyrases acts as targets in potential drug.
Objective: The article focuses on synthesis of [1,2,4]triazolo and [1,2,3,4]tetrazolo derivatives and their theoretical calculations that suggest they are anti-cancer substances.
Materials and Methods: DFT and computational studies were performed on the structural properties of experimental molecules experimentally, and significant theoretical calculations were performed based on density functional theory (DFT) with Becke’s three-parameter exchange function21-22 of correlation functional Lee Yang Parr (B3LYP) with the basis set 6-31G (d,p) using Gaussian 03 software23. Geometrical parameters of the optimized structures were calculated and also the charge on each atom (Mulliken charge). Chemcraft program24 was used to visualize the optimized structure and ChemBio3D ultra 12.0 was used to visualize the highest occupied and lowest unoccupied molecular orbitals.
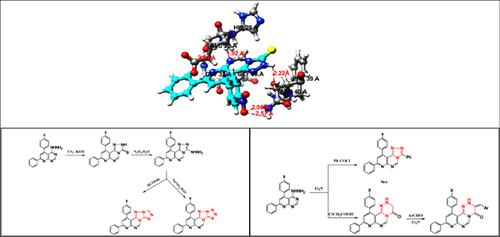
Results: Preliminary screening in five studied ligands acts as inhibitors for different active sites along the target. The molecular docking study also revealed that the compound 6c was the most effective compounds in inhibiting Tankyrase I enzyme (2rf5), this result can help strongly in inhibition of carcinogenic cells and cancer treatment.
Conclusion: We have described a new practical cyclocondensation synthesis for a series of [1,2,4]triazolo[4,3- c]pyrido[3,2-e] pyrimidine and pyrido[2',3':4,5] pyrimido[6,1-c][1,2,4] triazine from 2-amino-3-cyano-4.6- diarylpyridines. Also polyheterocyclic compounds containing [1,2,4]triazolo and [1,2,3,4]tetrazolo moieties were also synthesized through the reactions of 3-hydrazino-8,10-diaryl [1,2,4]triazolo[4,3-c]pyrido[3,2- e]pyrimidine with both formic acid and the formation of diazonuim salt respectively. Newly synthesized heterocycles structures were confirmed using elemental analysis, IR, 1H-NMR, 13C-NMR and mass spectral data. DFT and computational studies were carried out on five of the synthesized poly heterocyclic compounds to show their structural and geometrical parameters involved in the study. Molecular docking using Tankyrase I enzyme as a target showed how the studied heterocyclic compounds act as a ligand interacting most of active sites on Tankyrase I with a type of interactions specified for H-bonding and VDW. We investigated that the five studied ligands act as inhibitors for different active sites along the target. The molecular docking study also revealed that the compound 6c was the most effective compounds in inhibiting Tankyrase I enzyme (2rf5), this result can help strongly in inhibition of carcinogenic cells and cancer treatment.
中文翻译:

含[1,2,4]三唑和[1,2,3,4]四唑部分的多杂环化合物的新合成及其作为预期抗癌试剂的DFT研究。
背景:2-氨基-3-氰基吡啶是良好的起始试剂,已用于合成许多杂环化合物,例如吡啶并嘧啶,[1,2,4]三唑和[1,2,3,4]四唑衍生物,这些衍生物具有生物活性为抗微生物和细胞毒活性。同时,众所周知[1,2,4]三唑和[1,2,3,4]四唑衍生物具有许多生理活性,例如抗癌,抗真菌,肌肉松弛,催眠,抗炎,利尿和降压活性。已经研究了各种各样的杂环化合物以证明它们对DNA和RNA结构的生物活性。几种重要功能使Tankyrases成为潜在药物的靶标。
目的:本文重点研究[1,2,4]三唑和[1,2,3,4]四唑衍生物的合成及其理论计算,表明它们是抗癌物质。
材料与方法:对实验分子的结构特性进行了DFT和计算研究,并基于具有密度泛函理论(DFT)和相关功能的Lee Yang Parr(B3LYP)的Becke的三参数交换函数进行了重要的理论计算。 ),使用高斯03软件23.的基本设置为6-31G(d,p)。计算了优化结构的几何参数,还计算了每个原子上的电荷(Mulliken电荷)。使用Chemcraft程序24可视化优化的结构,使用ChemBio3D ultra 12.0可视化最高占据和最低未占据的分子轨道。
结果:在五个研究的配体中进行的初步筛选可作为沿靶标不同活性位点的抑制剂。分子对接研究还表明,化合物6c是抑制Tankyrase I酶(2rf5)的最有效化合物,该结果可强烈帮助抑制致癌细胞和治疗癌症。
结论:我们描述了一系列[1,2,4]三唑并[4,3-c]吡啶并[3,2-e]嘧啶和吡啶并[2',3':4,5]的新型实用的环缩合反应来自2-氨基-3-氰基-4.6-二芳基吡啶的嘧啶并[6,1-c] [1,2,4]三嗪。还通过3-肼基-8,10-二芳基[1,2,4]三唑[4]的反应合成了含有[1,2,4]三唑和[1,2,3,4]四唑部分的多杂环化合物。 ,3-c] pyrido [3,2-e] pyrimidine与甲酸和重氮盐形成。使用元素分析,IR,1H-NMR,13C-NMR和质谱数据确认了新合成的杂环结构。对五个合成的多杂环化合物进行了DFT和计算研究,以显示其参与研究的结构和几何参数。以Tankyrase I酶为目标的分子对接表明,所研究的杂环化合物如何以配体的形式与Tankyrase I上的大多数活性位点相互作用,并具有为H键和VDW指定的相互作用类型。我们调查了五个研究的配体充当沿目标不同活性位点的抑制剂。分子对接研究还表明,化合物6c是抑制Tankyrase I酶(2rf5)的最有效化合物,这一结果可大大有助于抑制致癌细胞和治疗癌症。我们调查了五个研究的配体充当沿目标不同活性位点的抑制剂。分子对接研究还表明,化合物6c是抑制Tankyrase I酶(2rf5)的最有效化合物,该结果可强烈帮助抑制致癌细胞和治疗癌症。我们调查了五个研究的配体充当沿目标不同活性位点的抑制剂。分子对接研究还表明,化合物6c是抑制Tankyrase I酶(2rf5)的最有效化合物,这一结果可大大有助于抑制致癌细胞和治疗癌症。











































 京公网安备 11010802027423号
京公网安备 11010802027423号