当前位置:
X-MOL 学术
›
J. Struct. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Higher-order oligomerization of a chimeric αβγ bifunctional diterpene synthase with prenyltransferase and class II cyclase activities is concentration-dependent.
Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.jsb.2020.107463 Trey A Ronnebaum 1 , Kushol Gupta 2 , David W Christianson 1
Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.jsb.2020.107463 Trey A Ronnebaum 1 , Kushol Gupta 2 , David W Christianson 1
Affiliation
|
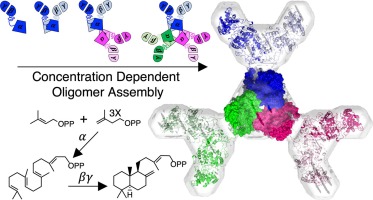
The unusual diterpene (C20) synthase copalyl diphosphate synthase from Penicillium verruculosum (PvCPS) is the first bifunctional terpene synthase identified with both prenyltransferase and class II cyclase activities in a single polypeptide chain with αβγ domain architecture. The C-terminal prenyltransferase α domain generates geranylgeranyl diphosphate which is then cyclized to form copalyl diphosphate at the N-terminal βγ domain interface. We now demonstrate that PvCPS exists as a hexamer at high concentrations - a unique quaternary structure for known αβγ terpene synthases. Hexamer assembly is corroborated by a 2.41 Å-resolution crystal structure of the α domain prenyltransferase obtained from limited proteolysis of full-length PvCPS, as well as the ab initio model of full-length PvCPS derived from small-angle X-ray scattering data. Hexamerization of the prenyltransferase α domain appears to drive the hexamerization of full-length PvCPS. The PvCPS hexamer dissociates into lower-order species at lower concentrations, as evidenced by size-exclusion chromatography in-line with multiangle light scattering, sedimentation velocity analytical ultracentrifugation, and native polyacrylamide gel electrophoresis experiments, suggesting that oligomerization is concentration dependent. Even so, PvCPS oligomer assembly does not affect prenyltransferase activity in vitro.
中文翻译:

具有异戊二烯基转移酶和II类环化酶活性的嵌合αβγ双功能二萜合酶的高级低聚是浓度依赖性的。
来自寻常青霉(PvCPS)的不寻常的二萜(C20)合酶椰油酰二磷酸合酶(PvCPS)是在具有αβγ域结构的单条多肽链中鉴定出异戊二烯基转移酶和II类环化酶活性的第一个双功能萜合酶。C末端异戊烯基转移酶α结构域生成香叶基香叶基二磷酸酯,然后将其环化以在N末端βγ结构域界面形成二棕榈酸二棕榈酯。现在,我们证明PvCPS以高浓度的六聚体形式存在-这是已知的αβγ萜烯合酶的独特四级结构。从全长PvCPS的有限蛋白水解获得的α结构域异戊二烯转移酶的2.41Å分辨率晶体结构,以及从小角度X射线散射数据得出的全长PvCPS的从头算模型,证实了六聚体组装。异戊二烯基转移酶α结构域的六聚化作用似乎驱动全长PvCPS的六聚化作用。PvCPS六聚体在较低浓度下会分解成低级物种,这通过在线排阻色谱,多角度光散射,沉降速度分析超速离心和天然聚丙烯酰胺凝胶电泳实验得以证明,表明低聚是浓度依赖性的。即便如此,PvCPS寡聚体组装仍不影响体外异戊二烯基转移酶的活性。和天然聚丙烯酰胺凝胶电泳实验,表明低聚是浓度依赖性的。即便如此,PvCPS寡聚体组装仍不影响体外异戊二烯基转移酶的活性。和天然聚丙烯酰胺凝胶电泳实验,表明低聚是浓度依赖性的。即便如此,PvCPS寡聚体组装仍不影响体外异戊二烯基转移酶的活性。
更新日期:2020-03-26
中文翻译:

具有异戊二烯基转移酶和II类环化酶活性的嵌合αβγ双功能二萜合酶的高级低聚是浓度依赖性的。
来自寻常青霉(PvCPS)的不寻常的二萜(C20)合酶椰油酰二磷酸合酶(PvCPS)是在具有αβγ域结构的单条多肽链中鉴定出异戊二烯基转移酶和II类环化酶活性的第一个双功能萜合酶。C末端异戊烯基转移酶α结构域生成香叶基香叶基二磷酸酯,然后将其环化以在N末端βγ结构域界面形成二棕榈酸二棕榈酯。现在,我们证明PvCPS以高浓度的六聚体形式存在-这是已知的αβγ萜烯合酶的独特四级结构。从全长PvCPS的有限蛋白水解获得的α结构域异戊二烯转移酶的2.41Å分辨率晶体结构,以及从小角度X射线散射数据得出的全长PvCPS的从头算模型,证实了六聚体组装。异戊二烯基转移酶α结构域的六聚化作用似乎驱动全长PvCPS的六聚化作用。PvCPS六聚体在较低浓度下会分解成低级物种,这通过在线排阻色谱,多角度光散射,沉降速度分析超速离心和天然聚丙烯酰胺凝胶电泳实验得以证明,表明低聚是浓度依赖性的。即便如此,PvCPS寡聚体组装仍不影响体外异戊二烯基转移酶的活性。和天然聚丙烯酰胺凝胶电泳实验,表明低聚是浓度依赖性的。即便如此,PvCPS寡聚体组装仍不影响体外异戊二烯基转移酶的活性。和天然聚丙烯酰胺凝胶电泳实验,表明低聚是浓度依赖性的。即便如此,PvCPS寡聚体组装仍不影响体外异戊二烯基转移酶的活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号