Journal of Contaminant Hydrology ( IF 3.5 ) Pub Date : 2020-01-22 , DOI: 10.1016/j.jconhyd.2020.103606 Joanna Kyziol-Komosinska 1 , Agnieszka Dzieniszewska 1 , Wojciech Franus 2 , Grzegorz Rzepa 3
|
In recent years, there has been a growth in the number of products containing Ag nanoparticles (AgNPs) in many areas and their use suggests that the water-soil environment may be exposed to the contaminant with different Ag species.
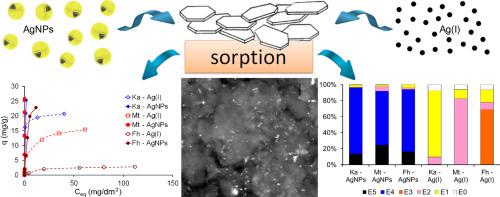
Therefore, the sorption of two Ag forms (i.e. Ag(I) ions and nanoparticles – AgNPs) on clay minerals (montmorillonite and kaolinite) and iron (oxyhydr)oxides (ferrihydrite) as a function of solution:mineral ratio (100:1, 250:1, 500:1), solution pH (3.0, 5.5 and 7.0) and initial Ag concentration (0.1–100 mg/dm3) was studied using batch method. In addition the binding strength/mobility of the bonded Ag species was researched. The results show a great sorption potential of clay minerals for both Ag forms and lower sorption capacity of ferrihydrite, in particular for Ag(I) ions. The maximum sorption capacities of montmorillonite, kaolinite and ferrihydrite estimated from three-parameter isotherm model of Sips were 94.39 mg/g, 117.8 mg/g and 26.48 mg/g for AgNPs and 17.92 mg/g, 21.14 mg/g and 3.072 mg/g for Ag(I) ions, respectively. Aggregation process plays an important role in sorption and mobility of AgNPs.
The sequential extraction study indicated different binding mechanisms of the Ag forms onto the clay minerals and ferrihydrite, which depended on the active sites of minerals as well as the Ag species nature in the solution. Ag(I) was weakly bound by clay minerals but presence of iron (oxyhydr)oxides decreased the Ag(I) mobility and bioavailability. On the other hand, AgNPs bound with the active centers of minerals in a very strong way and were not able to release into water.
The study of the binding of Ag forms by clay minerals and (oxyhydr)oxides allows to determine the influence of their physicochemical and structural properties, including e.g. pore size on Ag sorption. These results allow these properties to be taken into account in the study of environmental samples, including waters and soils.
Moreover, the results showed that in the study of behavior of Ag forms in contact with the minerals, in addition to the sorption capacity, the susceptibility to their release is very important.
Studies on sorption/desorption of AgNPs and Ag(I) ions as a form of oxidation of AgNPs is important for understanding the transport and fate of the Ag species in soil, sediments and surface water because of different their behavior in contact with the minerals.
中文翻译:

在水生沉积矿物质存在下,Ag物种的行为-在水生环境安全的背景下。
近年来,在许多地区,包含银纳米颗粒(AgNPs)的产品数量有所增长,其用途表明水土环境可能会暴露于具有不同Ag种类的污染物中。
因此,两种银的形式(即Ag(I)离子和纳米颗粒– AgNPs)在粘土矿物(蒙脱土和高岭石)和氧化铁(羟基氧化物)(亚铁酸盐)上的吸附随溶液:矿物比(100:1, 250:1、500:1),溶液pH(3.0、5.5和7.0)和初始Ag浓度(0.1–100 mg / dm 3))使用批处理方法进行了研究。另外,研究了键合的Ag物质的结合强度/迁移率。结果表明,粘土矿物对两种形式的银都有很大的吸附潜力,而对水铁矿,特别是对银离子的吸附能力较低。根据三参数等温等温线模型估计的蒙脱石,高岭石和水铁矿的最大吸附能力分别为:AgNPs分别为94.39 mg / g,117.8 mg / g和26.48 mg / g,17.92 mg / g,21.14 mg / g和3.072 mg / g g分别表示Ag(I)离子。聚集过程在AgNPs的吸附和迁移中起重要作用。
连续萃取研究表明,Ag形式与粘土矿物和水铁矿的结合机理不同,这取决于矿物的活性部位以及溶液中Ag的种类。Ag(I)与粘土矿物的结合较弱,但是氧化铁(羟基氧化物)的存在会降低Ag(I)的迁移率和生物利用度。另一方面,AgNPs以非常牢固的方式与矿物质的活性中心结合,无法释放到水中。
对粘土矿物和(羟基氧化物)氧化物与Ag形式结合的研究可以确定其物理化学和结构性质的影响,包括例如孔径对Ag吸附的影响。这些结果使得在研究包括水和土壤在内的环境样品时要考虑这些特性。
而且,结果表明,在研究银与矿物质接触的行为时,除了吸附能力外,其释放的敏感性也很重要。
研究AgNPs和Ag(I)离子作为AgNPs的一种氧化形式的吸附/解吸对于了解Ag物质在土壤,沉积物和地表水中的运输和结局非常重要,因为它们与矿物接触的行为不同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号