当前位置:
X-MOL 学术
›
Clin. Exp. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
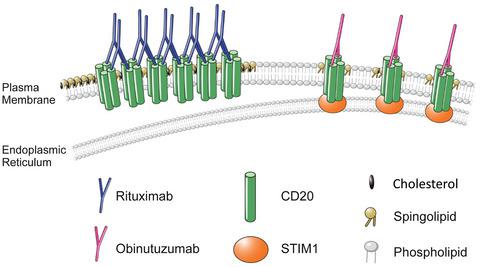
STIM1 knock-down decreases the affinity of obinutuzumab for CD20 by altering CD20 localization to Triton-soluble membrane.
Clinical & Experimental Immunology ( IF 4.6 ) Pub Date : 2020-02-21 , DOI: 10.1111/cei.13427 W Heo 1 , N Jin 1 , M S Park 1 , H-Y Kim 1 , S M Yoon 2 , J Lee 2 , J Y Kim 1
Clinical & Experimental Immunology ( IF 4.6 ) Pub Date : 2020-02-21 , DOI: 10.1111/cei.13427 W Heo 1 , N Jin 1 , M S Park 1 , H-Y Kim 1 , S M Yoon 2 , J Lee 2 , J Y Kim 1
Affiliation
|
Obinutuzumab is thought to exert its effects through its high antibody-dependent cellular cytotoxicity (ADCC) via glyco-engineering of the Fc region. In addition, obinutuzumab causes direct binding-induced cell death (DCD) only by specifically binding to its target CD20, a Ca2+ channel. However, the specific features of CD20 related to obinutuzumab binding-induction of cell death are not clearly understood. In this study, we evaluated the relationship between the Ca2+ channel features of CD20 as a store-operated Ca2+ channel (SOC) and obinutuzumab binding-induced cell death. Ca2+ channel function and biochemical analysis revealed that CD20 is an Orai1- and stromal interaction molecule (STIM1)-dependent Ca2+ pore. However, binding of obinutuzumab on CD20 did not have any effect on Ca2+ influx activity of CD20; the direct cell death rate mediated by obinutuzumab binding was almost equivalent with or without the extracellular Ca2+ condition. Given the apparent interaction between STIM1 and CD20, we observed Triton-X solubilized obinutuzumab-bound CD20 accompanied by STIM1. Subsequently, obinutuzumab binding and cell death were decreased by STIM1 knock-down in Ramos B cells. Thus, STIM1 directly contributes to cell death by increasing the affinity of cells for obinutuzumab by transferring CD20 to the Triton-soluble membrane region.
中文翻译:

STIM1敲低通过改变CD20在Triton可溶性膜上的定位来降低obinutuzumab对CD20的亲和力。
据认为,奥比努单抗通过其Fc区的糖工程改造,通过其高抗体依赖性细胞毒性(ADCC)发挥其作用。此外,obinutuzumab仅通过特异性结合其靶CD20(Ca2 +通道)而导致直接结合诱导的细胞死亡(DCD)。但是,尚不清楚与奥比妥珠单抗结合诱导细胞死亡有关的CD20的具体特征。在这项研究中,我们评估了CD20的Ca2 +通道特征作为存储操作的Ca2 +通道(SOC)与obinutuzumab结合诱导的细胞死亡之间的关系。Ca2 +通道功能和生化分析表明,CD20是Orai1和基质相互作用分子(STIM1)依赖的Ca2 +孔。然而,obinutuzumab与CD20的结合对CD20的Ca2 +内流活性没有任何影响。在有或没有细胞外Ca2 +条件下,由obinutuzumab结合介导的直接细胞死亡率几乎相等。给定STIM1和CD20之间的明显相互作用,我们观察到Triton-X溶解了与obututuzumab结合的CD20,并伴有STIM1。随后,拉莫斯B细胞中的STIM1敲低降低了obinutuzumab的结合和细胞死亡。因此,STIM1通过将CD20转移到Triton可溶性膜区域来增加细胞对obinutuzumab的亲和力,直接导致细胞死亡。
更新日期:2020-02-13
中文翻译:

STIM1敲低通过改变CD20在Triton可溶性膜上的定位来降低obinutuzumab对CD20的亲和力。
据认为,奥比努单抗通过其Fc区的糖工程改造,通过其高抗体依赖性细胞毒性(ADCC)发挥其作用。此外,obinutuzumab仅通过特异性结合其靶CD20(Ca2 +通道)而导致直接结合诱导的细胞死亡(DCD)。但是,尚不清楚与奥比妥珠单抗结合诱导细胞死亡有关的CD20的具体特征。在这项研究中,我们评估了CD20的Ca2 +通道特征作为存储操作的Ca2 +通道(SOC)与obinutuzumab结合诱导的细胞死亡之间的关系。Ca2 +通道功能和生化分析表明,CD20是Orai1和基质相互作用分子(STIM1)依赖的Ca2 +孔。然而,obinutuzumab与CD20的结合对CD20的Ca2 +内流活性没有任何影响。在有或没有细胞外Ca2 +条件下,由obinutuzumab结合介导的直接细胞死亡率几乎相等。给定STIM1和CD20之间的明显相互作用,我们观察到Triton-X溶解了与obututuzumab结合的CD20,并伴有STIM1。随后,拉莫斯B细胞中的STIM1敲低降低了obinutuzumab的结合和细胞死亡。因此,STIM1通过将CD20转移到Triton可溶性膜区域来增加细胞对obinutuzumab的亲和力,直接导致细胞死亡。



























 京公网安备 11010802027423号
京公网安备 11010802027423号