当前位置:
X-MOL 学术
›
Macromol. Biosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of 3D Printed Gelatin-Based Scaffolds with Varying Pore Size for MSC-Based Adipose Tissue Engineering.
Macromolecular Bioscience ( IF 4.4 ) Pub Date : 2020-02-20 , DOI: 10.1002/mabi.201900364 Liesbeth Tytgat 1, 2 , Matthias R Kollert 3, 4 , Lana Van Damme 1 , Hugo Thienpont 2 , Heidi Ottevaere 2 , Georg N Duda 3, 4 , Sven Geissler 3, 4 , Peter Dubruel 1 , Sandra Van Vlierberghe 1, 2 , Taimoor H Qazi 3, 4, 5
Macromolecular Bioscience ( IF 4.4 ) Pub Date : 2020-02-20 , DOI: 10.1002/mabi.201900364 Liesbeth Tytgat 1, 2 , Matthias R Kollert 3, 4 , Lana Van Damme 1 , Hugo Thienpont 2 , Heidi Ottevaere 2 , Georg N Duda 3, 4 , Sven Geissler 3, 4 , Peter Dubruel 1 , Sandra Van Vlierberghe 1, 2 , Taimoor H Qazi 3, 4, 5
Affiliation
|
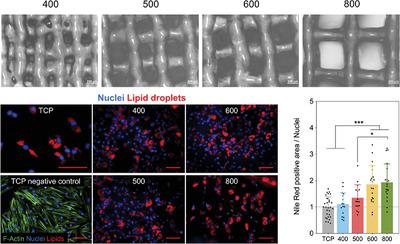
Adipose tissue engineering aims to provide solutions to patients who require tissue reconstruction following mastectomies or other soft tissue trauma. Mesenchymal stromal cells (MSCs) robustly differentiate into the adipogenic lineage and are attractive candidates for adipose tissue engineering. This work investigates whether pore size modulates adipogenic differentiation of MSCs toward identifying optimal scaffold pore size and whether pore size modulates spatial infiltration of adipogenically differentiated cells. To assess this, extrusion‐based 3D printing is used to fabricate photo‐crosslinkable gelatin‐based scaffolds with pore sizes in the range of 200–600 µm. The adipogenic differentiation of MSCs seeded onto these scaffolds is evaluated and robust lipid droplet formation is observed across all scaffold groups as early as after day 6 of culture. Expression of adipogenic genes on scaffolds increases significantly over time, compared to TCP controls. Furthermore, it is found that the spatial distribution of cells is dependent on the scaffold pore size, with larger pores leading to a more uniform spatial distribution of adipogenically differentiated cells. Overall, these data provide first insights into the role of scaffold pore size on MSC‐based adipogenic differentiation and contribute toward the rational design of biomaterials for adipose tissue engineering in 3D volumetric spaces.
中文翻译:

评估基于MSC的脂肪组织工程中孔径可变的3D打印明胶支架。
脂肪组织工程旨在为需要在乳房切除术或其他软组织创伤后进行组织重建的患者提供解决方案。间充质基质细胞(MSCs)强大地分化为成脂谱系,是脂肪组织工程的有吸引力的候选人。这项工作调查孔径是否调节MSC的成脂分化,以鉴定最佳支架孔径,以及孔径是否调节成脂分化细胞的空间浸润。为了评估这一点,使用基于挤出的3D打印来制造孔径在200–600 µm范围内的基于光交联明胶的支架。评估了接种在这些支架上的MSC的成脂分化,最早在培养的第6天后就在所有支架组中观察到了牢固的脂质滴形成。与TCP对照相比,随着时间的推移,支架上成脂基因的表达显着增加。此外,发现细胞的空间分布取决于支架的孔径,较大的孔导致成脂分化细胞的空间分布更均匀。总体而言,这些数据提供了关于支架孔径在基于MSC的成脂分化中作用的初步见解,并有助于合理设计3D容积空间中脂肪组织工程用生物材料。发现细胞的空间分布取决于支架的孔径,较大的孔导致成脂分化细胞的空间分布更均匀。总体而言,这些数据提供了关于支架孔径在基于MSC的成脂分化中作用的初步见解,并有助于合理设计3D体积空间中脂肪组织工程用生物材料。发现细胞的空间分布取决于支架的孔径,较大的孔导致成脂分化细胞的空间分布更均匀。总体而言,这些数据提供了关于支架孔径在基于MSC的成脂分化中作用的初步见解,并有助于合理设计3D容积空间中脂肪组织工程用生物材料。
更新日期:2020-02-20
中文翻译:

评估基于MSC的脂肪组织工程中孔径可变的3D打印明胶支架。
脂肪组织工程旨在为需要在乳房切除术或其他软组织创伤后进行组织重建的患者提供解决方案。间充质基质细胞(MSCs)强大地分化为成脂谱系,是脂肪组织工程的有吸引力的候选人。这项工作调查孔径是否调节MSC的成脂分化,以鉴定最佳支架孔径,以及孔径是否调节成脂分化细胞的空间浸润。为了评估这一点,使用基于挤出的3D打印来制造孔径在200–600 µm范围内的基于光交联明胶的支架。评估了接种在这些支架上的MSC的成脂分化,最早在培养的第6天后就在所有支架组中观察到了牢固的脂质滴形成。与TCP对照相比,随着时间的推移,支架上成脂基因的表达显着增加。此外,发现细胞的空间分布取决于支架的孔径,较大的孔导致成脂分化细胞的空间分布更均匀。总体而言,这些数据提供了关于支架孔径在基于MSC的成脂分化中作用的初步见解,并有助于合理设计3D容积空间中脂肪组织工程用生物材料。发现细胞的空间分布取决于支架的孔径,较大的孔导致成脂分化细胞的空间分布更均匀。总体而言,这些数据提供了关于支架孔径在基于MSC的成脂分化中作用的初步见解,并有助于合理设计3D体积空间中脂肪组织工程用生物材料。发现细胞的空间分布取决于支架的孔径,较大的孔导致成脂分化细胞的空间分布更均匀。总体而言,这些数据提供了关于支架孔径在基于MSC的成脂分化中作用的初步见解,并有助于合理设计3D容积空间中脂肪组织工程用生物材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号