Current Pharmaceutical Biotechnology ( IF 2.2 ) Pub Date : 2020-05-01 , DOI: 10.2174/1389201021666200217113049 Mohammed H Aldosari 1 , Marcel den Hartog 2 , Hubertina Ganizada 1 , Martijn J W Evers 1 , Enrico Mastrobattista 1 , Huub Schellekens 1
|
Objective: The high cost of orphan drugs limits their access by many patients, especially in low- and middle-income countries. Many orphan drugs are off-patent without alternative generic or biosimilar versions available. Production of these drugs at the point-of-care, when feasible, could be a cost-effective alternative.
Methods: The financial feasibility of this approach was estimated by setting up a small-scale production of recombinant human acid alpha-glucosidase (rhGAA). The commercial version of rhGAA is Myozyme™, and Lumizyme™ in the United States, which is used to treat Pompe disease. The rhGAA was produced in CHO-K1 mammalian cells and purified using multiple purification steps to obtain a protein profile comparable to Myozyme™.
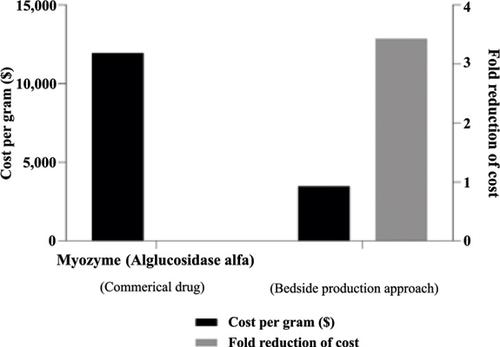
Results: The established small-scale production of rhGAA was used to obtain a realistic cost estimation for the magistral production of this biological drug. The treatment cost of rhGAA using bedside production was estimated at $3,484/gram, which is 71% lower than the commercial price of Myozyme ™.
Conclusion: This study shows that bedside production might be a cost-effective approach to increase the access of patients to particular life-saving drugs.
中文翻译:

重组人酸性α-葡萄糖苷酶在床边生产的可行性研究:技术和财务考虑。
目的:孤儿药的高昂费用限制了许多患者的使用,特别是在中低收入国家。许多孤儿药专利无效,没有其他可用的通用或生物仿制药版本。在可行的情况下,在护理点生产这些药物可能是一种经济高效的选择。
方法:通过建立重组人酸性α-葡萄糖苷酶(rhGAA)的小规模生产来评估此方法的财务可行性。rhGAA的商业版本是美国的Myozyme™和Lumizyme™,用于治疗庞贝病。rhGAA在CHO-K1哺乳动物细胞中产生,并使用多个纯化步骤进行纯化以获得与Myozyme™相当的蛋白质谱。
结果:rhGAA的既定小规模生产用于对该生物药物的原料生产进行现实的成本估算。使用床头生产的rhGAA的治疗成本估计为$ 3,484 / g,比Myozyme™的商业价格低71%。
结论:这项研究表明,床头生产可能是增加患者获得特定救生药物的成本效益的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号