Biologicals ( IF 1.5 ) Pub Date : 2020-02-06 , DOI: 10.1016/j.biologicals.2020.01.009 Sheethal Thomas Mannully 1 , V P B Rekha 2 , N Singh 2 , C Shanthi 1 , K K Pulicherla 3
|
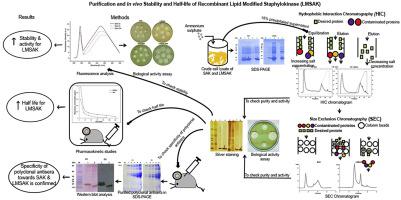
Staphylokinase (SAK), the thrombolytic protein holds a significant position in treating cardiovascular diseases. However, the rapid clearance of this protein from blood circulation reduces its effective usage and as a strategy to increase the half-life of SAK, initial work focussed on lipid modification of SAK (LMSAK) in E. coli GJ1158. Effective purification of the modified protein achieved using the two step method of hydrophobic interaction chromatography in succession with size exclusion chromatography, indicated a better yield. The thrombolytic activity of purified LMSAK analysed in heated plasma agar plate assay confirmed an enhanced activity. In vivo pharmacokinetic studies carried out for determining the half-life of LMSAK in blood circulation of mice presented that it has a half-life of 43.3 ± 3.4 min which is much higher than 21.6 ± 2.1 min that of the unmodified version of SAK. The studies confirmed the role of lipid modification as a crucial factor in confirming in vivo stability of LMSAK and proves to be beneficial in therapeutic usage.
中文翻译:

重组脂质修饰的葡萄激酶的纯化,体内稳定性和半衰期。
葡萄球激酶(SAK)是一种溶栓蛋白,在治疗心血管疾病中占有重要地位。然而,这种蛋白从血液循环中的快速清除降低了其有效使用量,并且作为增加SAK半衰期的策略,最初的工作集中在大肠杆菌GJ1158中SAK的脂质修饰(LMSAK)。使用疏水相互作用色谱法和尺寸排阻色谱法两步法实现的修饰蛋白的有效纯化表明收率更高。在加热的血浆琼脂平板分析中分析的纯化LMSAK的溶栓活性证实了活性的增强。体内用于确定LMSAK在小鼠血液循环中的半衰期的药代动力学研究表明,其半衰期为43.3±3.4分钟,远高于未修饰版SAK的21.6±2.1分钟。研究证实脂质修饰是确认LMSAK体内稳定性的关键因素,并被证明对治疗有用。










































 京公网安备 11010802027423号
京公网安备 11010802027423号