当前位置:
X-MOL 学术
›
Immunology
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Control of regulatory T-cell differentiation and function by T-cell receptor signalling and Foxp3 transcription factor complexes.
Immunology ( IF 4.9 ) Pub Date : 2020-03-09 , DOI: 10.1111/imm.13178 Masahiro Ono 1
Immunology ( IF 4.9 ) Pub Date : 2020-03-09 , DOI: 10.1111/imm.13178 Masahiro Ono 1
Affiliation
|
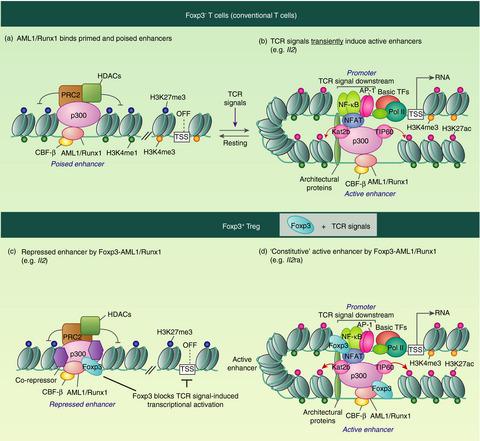
The transcription factor Foxp3 controls the differentiation and function of regulatory T-cells (Treg). Studies in the past decades identified numerous Foxp3-interacting protein partners. However, it is still not clear how Foxp3 produces the Treg-type transcriptomic landscape through cooperating with its partners. Here I show the current understanding of how Foxp3 transcription factor complexes regulate the differentiation, maintenance and functional maturation of Treg. Importantly, T-cell receptor (TCR) signalling plays central roles in Treg differentiation and Foxp3-mediated gene regulation. Differentiating Treg will have recognized their cognate antigens and received TCR signals before initiating Foxp3 transcription, which is triggered by TCR-induced transcription factors including NFAT, AP-1 and NF-κB. Once expressed, Foxp3 seizes TCR signal-induced transcriptional and epigenetic mechanisms through interacting with AML1/Runx1 and NFAT. Thus, Foxp3 modifies gene expression dynamics of TCR-induced genes, which constitute cardinal mechanisms for Treg-mediated immune suppression. Next, I discuss the following key topics, proposing new mechanistic models for Foxp3-mediated gene regulation: (i) how Foxp3 transcription is induced and maintained by the Foxp3-inducing enhanceosome and the Foxp3 autoregulatory transcription factor complex; (ii) molecular mechanisms for effector Treg differentiation (i.e. Treg maturation); (iii) how Foxp3 activates or represses its target genes through recruiting coactivators and corepressors; (iv) the 'decision-making' Foxp3-containing transcription factor complex for Th17 and Treg differentiation; and (v) the roles of post-translational modification in Foxp3 regulation. Thus, this article provides cutting-edge understanding of molecular biology of Foxp3 and Treg, integrating findings by biochemical and genomic studies.
中文翻译:

通过T细胞受体信号传导和Foxp3转录因子复合物控制调节性T细胞分化和功能。
转录因子Foxp3控制着调节性T细胞(Treg)的分化和功能。过去几十年的研究确定了许多与Foxp3相互作用的蛋白质伴侣。但是,尚不清楚Foxp3如何通过与其合作伙伴的合作产生Treg型转录组。在这里,我展示了对Foxp3转录因子复合物如何调节Treg的分化,维持和功能成熟的当前理解。重要的是,T细胞受体(TCR)信号在Treg分化和Foxp3介导的基因调控中起着核心作用。分化的Treg将在启动Foxp3转录之前识别它们的同源抗原并接收TCR信号,这是由TCR诱导的转录因子(包括NFAT,AP-1和NF-κB)触发的。表达后 Foxp3通过与AML1 / Runx1和NFAT相互作用,抓住了TCR信号诱导的转录和表观遗传机制。因此,Foxp3修改了TCR诱导的基因的基因表达动力学,这构成了Treg介导的免疫抑制的主要机制。接下来,我讨论以下关键主题,为Foxp3介导的基因调控提出新的机制模型:(i)Foxp3诱导增强体和Foxp3自调控转录因子复合物如何诱导和维持Foxp3转录;(ii)效应子Treg分化(即Treg成熟)的分子机制;(iii)Foxp3如何通过募集共激活因子和共核心因子来激活或抑制其靶基因;(iv)用于Th17和Treg分化的“决定性”含Foxp3转录因子复合物;(v)翻译后修饰在Foxp3调控中的作用。因此,本文提供了Foxp3和Treg分子生物学的前沿知识,并结合了生化和基因组研究的发现。
更新日期:2020-04-22
中文翻译:

通过T细胞受体信号传导和Foxp3转录因子复合物控制调节性T细胞分化和功能。
转录因子Foxp3控制着调节性T细胞(Treg)的分化和功能。过去几十年的研究确定了许多与Foxp3相互作用的蛋白质伴侣。但是,尚不清楚Foxp3如何通过与其合作伙伴的合作产生Treg型转录组。在这里,我展示了对Foxp3转录因子复合物如何调节Treg的分化,维持和功能成熟的当前理解。重要的是,T细胞受体(TCR)信号在Treg分化和Foxp3介导的基因调控中起着核心作用。分化的Treg将在启动Foxp3转录之前识别它们的同源抗原并接收TCR信号,这是由TCR诱导的转录因子(包括NFAT,AP-1和NF-κB)触发的。表达后 Foxp3通过与AML1 / Runx1和NFAT相互作用,抓住了TCR信号诱导的转录和表观遗传机制。因此,Foxp3修改了TCR诱导的基因的基因表达动力学,这构成了Treg介导的免疫抑制的主要机制。接下来,我讨论以下关键主题,为Foxp3介导的基因调控提出新的机制模型:(i)Foxp3诱导增强体和Foxp3自调控转录因子复合物如何诱导和维持Foxp3转录;(ii)效应子Treg分化(即Treg成熟)的分子机制;(iii)Foxp3如何通过募集共激活因子和共核心因子来激活或抑制其靶基因;(iv)用于Th17和Treg分化的“决定性”含Foxp3转录因子复合物;(v)翻译后修饰在Foxp3调控中的作用。因此,本文提供了Foxp3和Treg分子生物学的前沿知识,并结合了生化和基因组研究的发现。











































 京公网安备 11010802027423号
京公网安备 11010802027423号