Current Protein & Peptide Science ( IF 1.9 ) Pub Date : 2020-05-31 , DOI: 10.2174/1389203721666200204122732 Mohammad Furkan 1 , Rizwan Hasan Khan 1
|
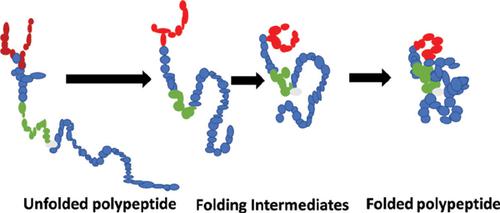
Protein folding is a natural phenomenon through which a linear polypeptide possessing necessary information attains three-dimension functionally active conformation. This is a complex and multistep process and therefore, the presence of several intermediary structures could be speculated as a result of protein folding. In in vivo, this folding process is governed by the assistance of other proteins called molecular chaperones and heat shock proteins. Due to the mechanism of protein folding, these intermediary structures remain major challenge for modern biology. Mutation in gene encoding amino acid can cause adverse environmental conditions which may result in misfolding of the linear polypeptide followed by the formation of aggregates and amyloidosis. Aggregation contributes to the pathophysiology of several maladies including diabetes mellitus, Huntington’s and Alzheimer’s disease. The propensity of native structure to form aggregated and fibrillar assemblies is a hallmark of amyloidosis. During aggregation of a protein, transition from α helix to β sheet is observed, and mainly β sheeted structure is visualised in a mature fibril. Heme proteins are very crucial for major life activities like transport of oxygen and carbon dioxide, synthesis of ATP, role in electron transport chain, and detoxification of free radicals formed during biochemical reactions. Any structural variation in the heme proteins may lead to a fatal response. Hence characterization of the folding intermediates becomes crucial. The characterization has been deciphered with the help of strong denaturants like acetonitrile and TFE. Moreover, possible role of elimination of these aggregates and prevention of protein denaturation is also discussed. Current review deals with the basic process and mechanism of the protein folding in general and the ultimate outcomes of the protein misfolding. Since Native conformation of heme proteins is essential for some vital activities as listed above, we have discussed possible prevention of denaturation and aggregation of heme proteins such as Hb, cyt c, catalase & peroxidase.
中文翻译:

血红蛋白的加工,结果和可能的聚集消除;可能对蛋白质病的治疗。
蛋白质折叠是一种自然现象,拥有必要信息的线性多肽可通过该现象获得三维功能活性构象。这是一个复杂且多步骤的过程,因此,可以推测由于蛋白质折叠而存在几种中间结构。在体内,这种折叠过程受称为分子伴侣蛋白和热激蛋白的其他蛋白的辅助。由于蛋白质折叠的机制,这些中间结构仍然是现代生物学的主要挑战。编码氨基酸的基因突变会引起不利的环境条件,可能导致线性多肽错误折叠,继而形成聚集体和淀粉样变性。聚集会导致多种疾病的病理生理,包括糖尿病,亨廷顿氏病和阿尔茨海默氏病。天然结构倾向于形成聚集的和纤维状的集合体是淀粉样变性病的标志。在蛋白质聚集期间,观察到从α螺旋向β折叠的转变,并且在成熟的原纤维中主要观察到β折叠的结构。血红素蛋白对于重要的生命活动至关重要,例如氧气和二氧化碳的运输,ATP的合成,在电子运输链中的作用以及生化反应过程中形成的自由基的解毒。血红素蛋白的任何结构变异都可能导致致命的反应。因此,折叠中间体的表征变得至关重要。在强变性剂(如乙腈和TFE)的帮助下破译了表征。此外,还讨论了消除这些聚集体和防止蛋白质变性的可能作用。当前的综述涉及蛋白质折叠的基本过程和机制以及蛋白质错误折叠的最终结果。由于血红素蛋白的天然构象对于上述某些重要活动至关重要,因此我们讨论了可能的预防血红素蛋白(如Hb,cyt c,过氧化氢酶和过氧化物酶)变性和聚集的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号