Current Organic Synthesis ( IF 1.7 ) Pub Date : 2020-02-29 , DOI: 10.2174/1570179417666200203121437 Zohreh Shahnavaz 1 , Lia Zaharani 1 , Mohd Rafie Johan 1 , Nader Ghaffari Khaligh 1
|
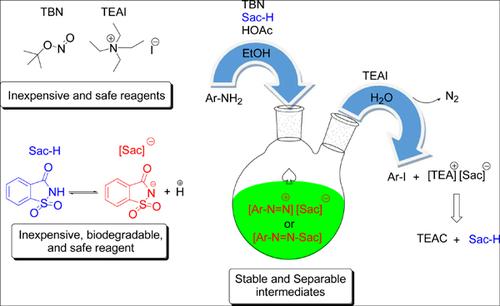
Background: In continuation of our previous work and the applications of saccharin, we encouraged to investigate the one-pot synthesis of the aryl iodides by the diazotization of the arene diazonium saccharin salts.
Objective: Arene diazonium salts play an important role in organic synthesis as intermediate and a wide variety of aromatic compounds have been prepared using them. A serious drawback of arene diazonium salts is their instability in a dry state; therefore, they must be stored and handled carefully to avoid spontaneous explosion and other hazard events.
Methods: The arene diazonium saccharin salts were prepared as active intermediates in situ through the reaction of various aryl amines with tert-butyl nitrite (TBN) in the presence of saccharin (Sac–H). Then, in situ obtained intermediates were used into the diazotization step without separation and purification in the current protocol.
Results: A variety of aryl iodides were synthesized at a greener and low-cost method in the presence of TBN, Sac–H, glacial acetic acid, and TEAI.
Conclusion: In summary, a telescopic reaction is developed for the synthesis of aryl iodides. The current methodology is safe, cost-effective, broad substrate scope, and metal-free. All used reagents are commercially available and inert to moisture and air. Also, the saccharine and tetraethylammonium cation could be partially recovered from the reaction residue, which reduces waste generation, energy consumption, raw material, and waste disposal costs.
中文翻译:

从芳族胺制备芳基碘化物的绿色替代品。
背景:在继续我们先前的工作和糖精的应用时,我们鼓励研究通过芳烃重氮盐精盐的重氮化一锅法合成芳基碘化物。
目的:芳烃重氮盐作为中间体在有机合成中起着重要作用,并已使用它们制备了多种芳族化合物。芳烃重氮盐的严重缺点是它们在干燥状态下不稳定。因此,必须小心存放和处理它们,以避免自发爆炸和其他危险事件。
方法:在糖精(Sac–H)存在下,通过各种芳基胺与亚硝酸叔丁酯(TBN)的反应,将芳烃重氮盐精盐原位制备为活性中间体。然后,原位获得的中间体无需进行分离和纯化即可用于重氮化步骤。
结果:在存在TBN,Sac–H,冰醋酸和TEAI的情况下,以更绿色,低成本的方法合成了多种芳基碘化物。
结论:总而言之,开发了一种伸缩反应用于芳基碘的合成。当前的方法是安全的,具有成本效益的,广泛的基材范围并且不含金属。所有使用的试剂都是可商购的,并且对湿气和空气呈惰性。此外,糖精和四乙铵阳离子可从反应残留物中部分回收,从而减少了废物产生,能源消耗,原材料和废物处理成本。









































 京公网安备 11010802027423号
京公网安备 11010802027423号