当前位置:
X-MOL 学术
›
Arch. Insect Biochem. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polycalin is involved in the toxicity and resistance to Cry1Ac toxin in Helicoverpa armigera (Hübner).
Archives of Insect Biochemistry and Physiology ( IF 1.5 ) Pub Date : 2020-02-03 , DOI: 10.1002/arch.21661 Bingjie Wang 1, 2 , Jizhen Wei 3 , Yanan Wang 2 , Lin Chen 2 , Gemei Liang 2
Archives of Insect Biochemistry and Physiology ( IF 1.5 ) Pub Date : 2020-02-03 , DOI: 10.1002/arch.21661 Bingjie Wang 1, 2 , Jizhen Wei 3 , Yanan Wang 2 , Lin Chen 2 , Gemei Liang 2
Affiliation
|
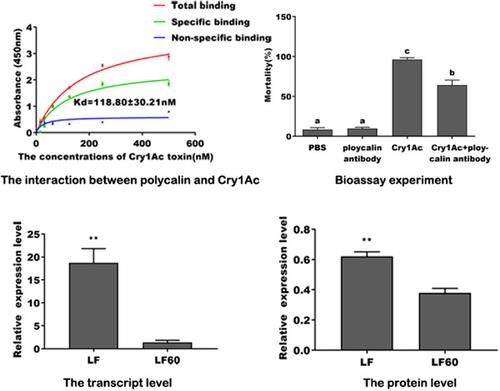
Polycalin has been confirmed as a binding protein of the Cry toxins in a few Lepidoptera insects, but its function in the action mechanism of Cry1Ac and whether it is involved in resistance evolution are still unclear. In this study, Ligand blot and enzyme‐linked immunosorbent assays showed that Helicoverpa armigera polycalin could specifically interact with Cry1Ac with a high affinity (Kd = 118.80 nM). Importantly, antisera blocking polycalin in H. armigera larvae decreased the toxicity of Cry1Ac by 31.84%. Furthermore, the relative gene and protein expressions were lower in Cry1Ac‐resistant strain (LF60) than that in Cry1Ac‐susceptible strain (LF). These findings indicated that H. armigera polycalin was a possible receptor of Cry1Ac and may be contributed to the resistance to Cry1Ac.
中文翻译:

Polycalin参与了棉铃虫对Hry1Ac毒素的毒性和抗性研究。
在许多鳞翅目昆虫中,Polycalin已被证实是Cry毒素的结合蛋白,但其在Cry1Ac的作用机制中的功能及其是否参与抗性进化仍不清楚。在这项研究中,配体印迹和酶联免疫吸附试验表明,棉铃虫聚卡林可以与Cry1Ac特异性相互作用,亲和力高(K d = 118.80 nM)。重要的是,棉铃虫幼虫中的抗血清阻断多卡林可将Cry1Ac的毒性降低31.84%。此外,耐Cry1Ac的菌株(LF60)的相对基因和蛋白质表达低于耐Cry1Ac的菌株(LF)。这些发现表明棉铃虫 polycalin是Cry1Ac的可能受体,可能对Cry1Ac具有抗性。
更新日期:2020-02-03
中文翻译:

Polycalin参与了棉铃虫对Hry1Ac毒素的毒性和抗性研究。
在许多鳞翅目昆虫中,Polycalin已被证实是Cry毒素的结合蛋白,但其在Cry1Ac的作用机制中的功能及其是否参与抗性进化仍不清楚。在这项研究中,配体印迹和酶联免疫吸附试验表明,棉铃虫聚卡林可以与Cry1Ac特异性相互作用,亲和力高(K d = 118.80 nM)。重要的是,棉铃虫幼虫中的抗血清阻断多卡林可将Cry1Ac的毒性降低31.84%。此外,耐Cry1Ac的菌株(LF60)的相对基因和蛋白质表达低于耐Cry1Ac的菌株(LF)。这些发现表明棉铃虫 polycalin是Cry1Ac的可能受体,可能对Cry1Ac具有抗性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号