Current Computer-Aided Drug Design ( IF 1.5 ) Pub Date : 2021-03-31 , DOI: 10.2174/1573409916666200131142619 N Ramalakshmi 1 , S R Chitra 1 , P Manimegalai 1 , S Arunkumar 2
|
Background: Hospital-acquired (HA) infections are caused due to E. coli, which is resistant to multiple drugs particularly to fluoroquinolone class of drugs. Urinary tract infections (UTI) affects people in the community and hospitals. 150 million people per annum are suffering from UTI worldwide.
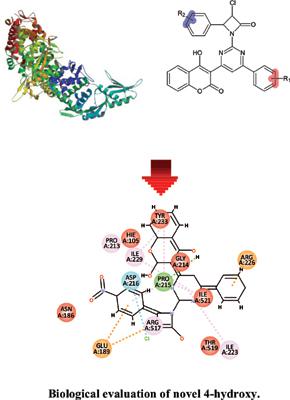
Methods: In this present study, we designed 36 novel coumarin derivatives, also we predicted pharmacokinetic and toxicity parameters. Docking studies were also carried out and all the compounds were evaluated for antibacterial activity against resistant quinolone E. coli strain ATCC 25922. It was interesting to note that the introduction of electron-withdrawing group on the aromatic ring resulted in compounds with an increased antibacterial activity, which is observed in compound 6 (with 4-nitro substitution), compound 23 (chloro) and compound 30 (chloro, nitro).
Results: From the MIC results, it was observed that compounds 6, 23 and 30 showed higher activity with 0.5μg/ml, 0. 12 μg/ml, 0.5 μg/ml respectively. Docking studies were performed with the active site of DNA gyrase (PDB ID: 4CKK). The maximum binding energy was found to be -10.7 Kcal/mol.
Conclusion: From the study, it was found that 3 compounds were potentially active against quinolone- resistant E. coli strains. This study can further be extended for in vivo evaluation.
中文翻译:

新型 4-羟基香豆素衍生物的设计、合成、对接和生物学评价
背景:医院获得性 (HA) 感染是由大肠杆菌引起的,大肠杆菌对多种药物特别是氟喹诺酮类药物具有耐药性。尿路感染 (UTI) 会影响社区和医院的人们。全世界每年有 1.5 亿人患有 UTI。
方法:在本研究中,我们设计了 36 种新型香豆素衍生物,并预测了药代动力学和毒性参数。还进行了对接研究,并评估了所有化合物对耐药喹诺酮类大肠杆菌菌株 ATCC 25922 的抗菌活性。有趣的是,在芳环上引入吸电子基团导致化合物抗菌活性增加,这在化合物 6(具有 4-硝基取代)、化合物 23(氯)和化合物 30(氯,硝基)中观察到。
结果:从MIC结果可以看出,化合物6、23和30分别在0.5μg/ml、0. 12μg/ml、0.5μg/ml时表现出较高的活性。对接研究是用 DNA 促旋酶的活性位点 (PDB ID: 4CKK) 进行的。发现最大结合能为-10.7 Kcal/mol。
结论:从该研究中,发现 3 种化合物对耐喹诺酮类大肠杆菌菌株具有潜在活性。这项研究可以进一步扩展到体内评估。











































 京公网安备 11010802027423号
京公网安备 11010802027423号