Protein & Peptide Letters ( IF 1.0 ) Pub Date : 2020-07-31 , DOI: 10.2174/0929866527666200129141116 Abdul Majid 1 , Farah Naz 1 , Muhammad Hassan Khaskheli 1
|
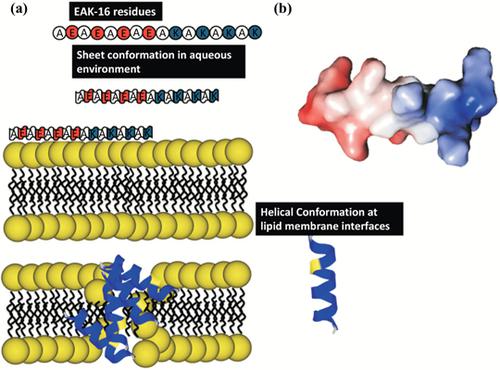
Background: Ionic complementary peptide EAK-16 has been studies for anticancer drug delivery application. This is a 16 residues, short sequence peptide has ability to trosnform into micro/nanoparticle via self-assembly. However, it is still not clear that how this can bind with cell membrane to induce membrane leakage or delivering their cargo inside cell membrane.
Objective: The main objective of this work was to understand behaviour of secondary structure conformation of peptide in solution and at lipid membrane interfaces and membrane permeability of synthetic ionic complementary peptide EAK-16. The corresponding secondary structure conformation was evaluated.
Methods: We performed biophysical investigation to probe the interaction of synthesised ionic complementary peptide (EAK-16) with dimyristoylphospholcholine (DMPC) and dimyristoylphosphoserine (DMPS) membrane interfaces. The folding behaviours of EAK-16 were studied with Circular Dichroism (CD) spectroscopy. Membrane leakage with peptide was confirmed with calcein leakage assay.
Results: Our finding of this study showed that in aqueous phase EAK-16 was predominantly folded into β-sheets. The temperature could alter the β-sheets. However, in DMPC and DMPS membrane interfaces, EAK-16 adopted helical conformation. EAK-16 has preference in perturbing anionic compared Zwitterionic lipid vesicles. This study proposed that hydrophobic grooves of EAK-16 might be a key in the association with lipid bilayers. Secondly, a charge distribution of ionic residues would also support the orientation at lipid bilayers. This peptide membrane association would facilitate the membrane destabilisation.
Conclusion: This study demonstrated the supporting evidence that EAK-16 could interact with lipid membranes and conforming to helical structure, while the helical conformation induced the lipid membrane leakage. Overall, this study provides a physical rationale that ionic complementary peptide can be a useful tool for designing and development of novel antibiotics and anticancer agents along its previous drug delivery applications.
中文翻译:

EAK-16肽诱导囊泡膜泄漏的结构可塑性。
背景:离子互补肽EAK-16已在抗癌药物递送应用中进行了研究。这是一个16个残基的短序列肽,具有通过自组装转化为微/纳米颗粒的能力。但是,尚不清楚它如何与细胞膜结合以引起膜泄漏或将其货物递送到细胞膜内。
目的:这项工作的主要目的是了解肽在溶液中和在脂质膜界面的二级结构构象的行为以及合成离子互补肽EAK-16的膜通透性。评价了相应的二级结构构象。
方法:我们进行了生物物理研究,以探测合成的离子互补肽(EAK-16)与二肉豆蔻基磷酸胆碱(DMPC)和二肉豆蔻基磷酸丝氨酸(DMPS)膜界面之间的相互作用。EAK-16的折叠行为用圆二色性(CD)光谱进行了研究。用钙黄绿素泄漏测定法证实了肽的膜泄漏。
结果:我们对这项研究的发现表明,EAK-16在水相中主要折叠成β-折叠。温度可能会改变β-折叠。但是,在DMPC和DMPS膜界面中,EAK-16采用了螺旋构象。EAK-16在干扰阴离子比较的两性离子脂质囊泡方面具有优势。这项研究提出,EAK-16的疏水沟可能是与脂质双层结合的关键。其次,离子残基的电荷分布也将支持脂质双层的取向。这种肽膜结合将促进膜去稳定。
结论:本研究证明了EAK-16可以与脂质膜相互作用并符合螺旋结构,而螺旋构象却引起脂质膜渗漏的支持证据。总体而言,这项研究提供了一种物理原理,即离子互补肽可以作为沿其以前的药物递送应用设计和开发新型抗生素和抗癌剂的有用工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号