当前位置:
X-MOL 学术
›
J. Struct. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structures of catalytic cycle intermediates of the Pyrococcus furiosus methionine adenosyltransferase demonstrate negative cooperativity in the archaeal orthologues.
Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-01-18 , DOI: 10.1016/j.jsb.2020.107462 Claudia Minici 1 , Laura Mosca 2 , Concetta Paola Ilisso 2 , Giovanna Cacciapuoti 2 , Marina Porcelli 2 , Massimo Degano 1
Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-01-18 , DOI: 10.1016/j.jsb.2020.107462 Claudia Minici 1 , Laura Mosca 2 , Concetta Paola Ilisso 2 , Giovanna Cacciapuoti 2 , Marina Porcelli 2 , Massimo Degano 1
Affiliation
|
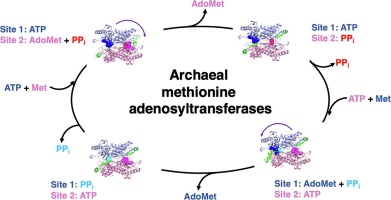
Methionine adenosyltransferases catalyse the biosynthesis of S-adenosylmethionine, the primary methyl group donor in biochemical reactions, through the condensation of methionine and ATP. Here, we report the structural analysis of the Pyrococcus furiosus methionine adenosyltransferase (PfMAT) captured in the unliganded, substrate- and product-bound states. The conformational changes taking place during the enzymatic catalytic cycle are allosterically propagated by amino acid residues conserved in the archaeal orthologues to induce an asymmetric dimer structure. The distinct occupancy of the active sites within a PfMAT dimer is consistent with a half-site reactivity that is mediated by a product-induced negative cooperativity. The structures of intermediate states of PfMAT reported here suggest a distinct molecular mechanism for S-adenosylmethionine synthesis in Archaea, likely consequence of the evolutionary pressure to achieve protein stability under extreme conditions.
中文翻译:

激烈热球菌蛋氨酸腺苷基转移酶的催化循环中间体的结构在古细菌直系同源物中显示出负的协同作用。
蛋氨酸腺苷基转移酶通过蛋氨酸和ATP的缩合催化生化反应中主要的甲基供体S-腺苷蛋氨酸的生物合成。在这里,我们报告在激烈的,基质和产品绑定状态捕获的激烈热球菌蛋氨酸腺苷基转移酶(PfMAT)的结构分析。在酶催化循环中发生的构象变化被古细菌直向同源物中保守的氨基酸残基变构地传播,以诱导不对称的二聚体结构。PfMAT二聚体中活性位点的独特占据与由产物诱导的负协同性介导的半位反应性一致。
更新日期:2020-03-26
中文翻译:

激烈热球菌蛋氨酸腺苷基转移酶的催化循环中间体的结构在古细菌直系同源物中显示出负的协同作用。
蛋氨酸腺苷基转移酶通过蛋氨酸和ATP的缩合催化生化反应中主要的甲基供体S-腺苷蛋氨酸的生物合成。在这里,我们报告在激烈的,基质和产品绑定状态捕获的激烈热球菌蛋氨酸腺苷基转移酶(PfMAT)的结构分析。在酶催化循环中发生的构象变化被古细菌直向同源物中保守的氨基酸残基变构地传播,以诱导不对称的二聚体结构。PfMAT二聚体中活性位点的独特占据与由产物诱导的负协同性介导的半位反应性一致。










































 京公网安备 11010802027423号
京公网安备 11010802027423号