Current Organic Synthesis ( IF 1.7 ) Pub Date : 2020-02-29 , DOI: 10.2174/1570179417666200124103400 Min-Xin Li 1 , Xiao-Jia Pu 1 , Xia Zhang 1 , Xi Zheng 2 , Hui Gao 1 , Wei-Lie Xiao 3 , Chun-Ping Wan 2 , Ze-Wei Mao 1
|
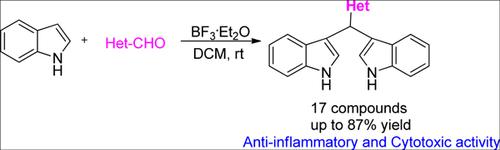
Background: Bis(indolyl)methane derivatives are widely found in nature with a broad range of biological and pharmacological activities. The development of techniques for the synthesis and functionalization of bis(indolyl)methanes have attracted more and more attention in recent years.
Objective: To study the synthesis and biological activity of heterocyclic substituted bis(indolyl)methanes.
Materials and Methods: A series of heterocyclic substituted bis(indolyl)methanes (3a-3p) have been prepared by condensation reaction of indole and heterocyclic aldehydes catalyzed by boron trifluoride etherate with high yields. Preliminary in vitro anti-inflammatory in lipopolysaccharide (LPS)-stimulated RAW-264.7 macrophages and cytotoxic activity against human tumor cell lines (A549, Hela and SGC7901) by MTT assay were tested.
Results: The result indicated that heterocyclic substituted bis(indolyl)methanes showed good antiinflammatory and selective cytotoxic activity. Especially, compounds 3o, 3p and 3q displayed similar inhibitory effect on the generation of NO to positive control dexamethasone, and compound 3q displayed similar selective cytotoxic activity to 5-FU.
Conclusion: Heterocyclic substituted bis(indolyl)methanes may be used as potential anti-inflammatory and anticancer leads.
中文翻译:

杂环取代的双(吲哚基)甲烷的合成及生物评价。
背景:双(吲哚基)甲烷衍生物在自然界中被广泛发现,具有广泛的生物学和药理活性。近年来,双(吲哚基)甲烷的合成和功能化技术的发展引起了越来越多的关注。
目的:研究杂环取代的双(吲哚基)甲烷的合成及生物活性。
材料与方法:通过三氟化硼醚化物催化的吲哚与杂环醛的缩合反应,制备了一系列杂环取代的双(吲哚基)甲烷(3a-3p)。通过MTT法测试了脂多糖(LPS)刺激的RAW-264.7巨噬细胞的初步体外抗炎作用和对人肿瘤细胞系(A549,Hela和SGC7901)的细胞毒活性。
结果:结果表明,杂环取代的双(吲哚基)甲烷具有良好的抗炎和选择性的细胞毒活性。特别地,化合物3o,3p和3q对NO的产生显示出与阳性对照地塞米松相似的抑制作用,并且化合物3q显示出与5-FU相似的选择性细胞毒活性。
结论:杂环取代的双(吲哚基)甲烷可用作潜在的抗炎和抗癌药。











































 京公网安备 11010802027423号
京公网安备 11010802027423号