当前位置:
X-MOL 学术
›
Arch. Insect Biochem. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of FerLCH, isolation of ferritin and functional analysis related to interaction with pathogens in Eri-silkworm, Samia cynthia ricini.
Archives of Insect Biochemistry and Physiology ( IF 1.5 ) Pub Date : 2020-01-24 , DOI: 10.1002/arch.21659 Li-Ang Yang 1, 2 , Jie Wang 1, 2 , Shahzad Toufeeq 1, 2 , Lin-Bao Zhu 1, 2 , Shang-Zhi Zhang 1, 2 , Ling-Ling You 1, 2 , Pei Hu 1, 2 , Hai-Zhong Yu 1, 2 , Kang Zhao 1, 2 , Xin Xu 1, 2 , Jia-Ping Xu 1, 2
Archives of Insect Biochemistry and Physiology ( IF 1.5 ) Pub Date : 2020-01-24 , DOI: 10.1002/arch.21659 Li-Ang Yang 1, 2 , Jie Wang 1, 2 , Shahzad Toufeeq 1, 2 , Lin-Bao Zhu 1, 2 , Shang-Zhi Zhang 1, 2 , Ling-Ling You 1, 2 , Pei Hu 1, 2 , Hai-Zhong Yu 1, 2 , Kang Zhao 1, 2 , Xin Xu 1, 2 , Jia-Ping Xu 1, 2
Affiliation
|
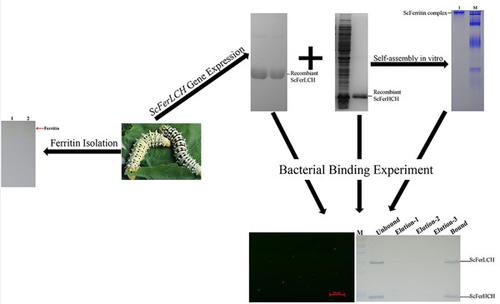
Ferritin is a ubiquitous and conserved iron storage protein that plays a significant role in host detoxification, iron storage, and immune response. Although ferritin has been studied in many species, little is known about its role in the Eri‐silkworm (Samia cynthia ricini). In this study, the ferritin light‐chain subunit gene, named ScFerLCH, was identified from S. c. ricini. The full‐length gene, ScFerLCH, was 1,155 bp and encoded a protein consisting of 231 amino acids with a deduced molecular weight of 26.38 kDa. Higher ScFerLCH expression levels were found in the midgut, silk gland, and fat body by quantitative reverse‐transcription polymerase chain reaction and western blot analysis. Injection of Staphylococcus aureus and Pseudomonas aeruginosa could induce upregulation of ScFerLCH in the hemolymph, fat body, and midgut, indicating that ScFerLCH may contribute to the host defense against invading pathogens. In addition, the native ferritin protein was isolated from S. c. ricini by native polyacrylamide gel electrophoresis and its two subunits, ferritin heavy‐chain subunit (ScFerHCH) and ferritin light‐chain subunit (ScFerLCH), were identified by mass spectrometry. Specifically, we found that recombinant ferritin subunits could self‐assemble into a protein complex in vitro; moreover, both recombinant subunits and the protein complex were found to bind different bacteria, including Escherichia coli, P. aeruginosa, S. aureus, and Bacillus subtilis. However, bactericidal tests showed that the protein complex could not inhibit the growth of bacteria directly. Taken together, our results suggest that ScFerritin might play an important role in mediating molecular interaction with pathogens.
中文翻译:

FerLCH的鉴定,铁蛋白的分离以及功能性分析与桑蚕辛里克虫的病原体相互作用。
铁蛋白是一种普遍存在且保守的铁存储蛋白,在宿主排毒,铁存储和免疫反应中起重要作用。虽然铁蛋白在许多物种进行了研究,鲜为人知的是,它在蓖麻蚕(角色萨米亚蚕)。在这项研究中,铁蛋白轻链亚基基因被命名为ScFerLCH 。里奇尼。全长基因ScFerLCH为1,155 bp,编码一种由231个氨基酸组成的蛋白质,推导分子量为26.38 kDa。通过定量逆转录聚合酶链反应和蛋白质印迹分析,在中肠,丝腺和脂肪体中发现较高的ScFerLCH表达水平。注入金黄色葡萄球菌和铜绿假单胞菌可诱导血淋巴,脂肪体和中肠中ScFerLCH的上调,表明ScFerLCH可能有助于宿主防御入侵的病原体。另外,天然的铁蛋白蛋白是从S.c.分离得到的。通过天然聚丙烯酰胺凝胶电泳对蓖麻毒素进行分析,并通过质谱法鉴定了蓖麻毒素及其两个亚基:铁蛋白重链亚基(ScFerHCH)和铁蛋白轻链亚基(ScFerLCH)。特别是,我们发现重组铁蛋白亚基可以在体外自组装成蛋白质复合物。此外,发现重组亚基和蛋白质复合物都结合不同的细菌,包括大肠杆菌,铜绿假单胞菌,金黄色葡萄球菌和枯草芽孢杆菌。但是,杀菌试验表明该蛋白质复合物不能直接抑制细菌的生长。两者合计,我们的结果表明ScFerritin可能在介导与病原体的分子相互作用中起重要作用。
更新日期:2020-01-24
中文翻译:

FerLCH的鉴定,铁蛋白的分离以及功能性分析与桑蚕辛里克虫的病原体相互作用。
铁蛋白是一种普遍存在且保守的铁存储蛋白,在宿主排毒,铁存储和免疫反应中起重要作用。虽然铁蛋白在许多物种进行了研究,鲜为人知的是,它在蓖麻蚕(角色萨米亚蚕)。在这项研究中,铁蛋白轻链亚基基因被命名为ScFerLCH 。里奇尼。全长基因ScFerLCH为1,155 bp,编码一种由231个氨基酸组成的蛋白质,推导分子量为26.38 kDa。通过定量逆转录聚合酶链反应和蛋白质印迹分析,在中肠,丝腺和脂肪体中发现较高的ScFerLCH表达水平。注入金黄色葡萄球菌和铜绿假单胞菌可诱导血淋巴,脂肪体和中肠中ScFerLCH的上调,表明ScFerLCH可能有助于宿主防御入侵的病原体。另外,天然的铁蛋白蛋白是从S.c.分离得到的。通过天然聚丙烯酰胺凝胶电泳对蓖麻毒素进行分析,并通过质谱法鉴定了蓖麻毒素及其两个亚基:铁蛋白重链亚基(ScFerHCH)和铁蛋白轻链亚基(ScFerLCH)。特别是,我们发现重组铁蛋白亚基可以在体外自组装成蛋白质复合物。此外,发现重组亚基和蛋白质复合物都结合不同的细菌,包括大肠杆菌,铜绿假单胞菌,金黄色葡萄球菌和枯草芽孢杆菌。但是,杀菌试验表明该蛋白质复合物不能直接抑制细菌的生长。两者合计,我们的结果表明ScFerritin可能在介导与病原体的分子相互作用中起重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号