当前位置:
X-MOL 学术
›
STEM CELLS
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Genome-Wide CRISPR Screen Identifies ZIC2 As An Essential Gene That Controls The Cell-Fate of Early Mesodermal Precursors To Human Heart Progenitors
STEM CELLS ( IF 4.0 ) Pub Date : 2020-03-10 , DOI: 10.1002/stem.3168 Jiejia Xu 1 , Chikai Zhou 1 , Kylie S Foo 2 , Ran Yang 2 , Yao Xiao 1 , Kristine Bylund 2 , Makoto Sahara 1, 2 , Kenneth R Chien 1, 2
STEM CELLS ( IF 4.0 ) Pub Date : 2020-03-10 , DOI: 10.1002/stem.3168 Jiejia Xu 1 , Chikai Zhou 1 , Kylie S Foo 2 , Ran Yang 2 , Yao Xiao 1 , Kristine Bylund 2 , Makoto Sahara 1, 2 , Kenneth R Chien 1, 2
Affiliation
|
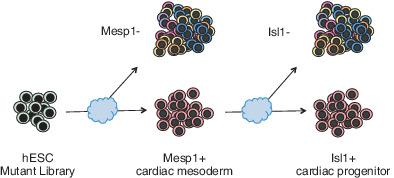
Cardiac progenitor formation is one of the earliest committed steps of human cardiogenesis and requires the cooperation of multiple gene sets governed by developmental signaling cascades. To determine the key regulators for cardiac progenitor formation, we have developed a two‐stage genome‐wide CRISPR‐knockout screen. We mimicked the progenitor formation process by differentiating human pluripotent stem cells (hPSCs) into cardiomyocytes, monitored by two distinct stage markers of early cardiac mesodermal formation and commitment to a multipotent heart progenitor cell fate: MESP1 and ISL1, respectively. From the screen output, we compiled a list of 15 candidate genes. After validating seven of them, we identified ZIC2 as an essential gene for cardiac progenitor formation. ZIC2 is known as a master regulator of neurogenesis. hPSCs with ZIC2 mutated still express pluripotency markers. However, their ability to differentiate into cardiomyocytes was greatly attenuated. RNA‐Seq profiling of the ZIC2‐mutant cells revealed that the mutants switched their cell fate alternatively to the noncardiac cell lineage. Further, single cell RNA‐seq analysis showed the ZIC2 mutants affected the apelin receptor‐related signaling pathway during mesoderm formation. Our results provide a new link between ZIC2 and human cardiogenesis and document the potential power of a genome‐wide unbiased CRISPR‐knockout screen to identify the key steps in human mesoderm precursor cell‐ and heart progenitor cell‐fate determination during in vitro hPSC cardiogenesis.
中文翻译:

全基因组 CRISPR 筛选将 ZIC2 鉴定为控制人类心脏祖细胞早期中胚层前体细胞命运的基本基因
心脏祖细胞的形成是人类心脏发生最早的承诺步骤之一,需要由发育信号级联控制的多个基因组的合作。为了确定心脏祖细胞形成的关键调节因子,我们开发了一个两阶段的全基因组 CRISPR 敲除筛选。我们通过将人类多能干细胞 (hPSC) 分化为心肌细胞来模拟祖细胞形成过程,通过早期心脏中胚层形成的两个不同阶段标志物和对多能心脏祖细胞命运的承诺进行监测:分别为 MESP1 和 ISL1。从屏幕输出中,我们编制了 15 个候选基因的列表。在验证了其中的七个后,我们将 ZIC2 鉴定为心脏祖细胞形成的必需基因。ZIC2 被称为神经发生的主要调节因子。ZIC2 突变的 hPSC 仍表达多能性标记。然而,它们分化成心肌细胞的能力大大减弱。ZIC2 突变细胞的 RNA-Seq 分析显示,突变体将它们的细胞命运交替转换为非心脏细胞谱系。此外,单细胞 RNA-seq 分析表明,ZIC2 突变体在中胚层形成过程中影响了 apelin 受体相关的信号通路。我们的结果提供了 ZIC2 与人类心脏发生之间的新联系,并记录了全基因组无偏 CRISPR 敲除筛选在体外 hPSC 心脏发生过程中确定人类中胚层前体细胞和心脏祖细胞命运的关键步骤的潜在能力。ZIC2 突变细胞的 RNA-Seq 分析显示,突变体将它们的细胞命运交替转换为非心脏细胞谱系。此外,单细胞 RNA-seq 分析表明,ZIC2 突变体在中胚层形成过程中影响了 apelin 受体相关的信号通路。我们的结果提供了 ZIC2 与人类心脏发生之间的新联系,并记录了全基因组无偏 CRISPR 敲除筛选在体外 hPSC 心脏发生过程中确定人类中胚层前体细胞和心脏祖细胞命运的关键步骤的潜在能力。ZIC2 突变细胞的 RNA-Seq 分析显示,突变体将它们的细胞命运交替转换为非心脏细胞谱系。此外,单细胞 RNA-seq 分析表明,ZIC2 突变体在中胚层形成过程中影响了 apelin 受体相关的信号通路。我们的结果提供了 ZIC2 与人类心脏发生之间的新联系,并记录了全基因组无偏 CRISPR 敲除筛选在体外 hPSC 心脏发生过程中确定人类中胚层前体细胞和心脏祖细胞命运的关键步骤的潜在能力。
更新日期:2020-03-10
中文翻译:

全基因组 CRISPR 筛选将 ZIC2 鉴定为控制人类心脏祖细胞早期中胚层前体细胞命运的基本基因
心脏祖细胞的形成是人类心脏发生最早的承诺步骤之一,需要由发育信号级联控制的多个基因组的合作。为了确定心脏祖细胞形成的关键调节因子,我们开发了一个两阶段的全基因组 CRISPR 敲除筛选。我们通过将人类多能干细胞 (hPSC) 分化为心肌细胞来模拟祖细胞形成过程,通过早期心脏中胚层形成的两个不同阶段标志物和对多能心脏祖细胞命运的承诺进行监测:分别为 MESP1 和 ISL1。从屏幕输出中,我们编制了 15 个候选基因的列表。在验证了其中的七个后,我们将 ZIC2 鉴定为心脏祖细胞形成的必需基因。ZIC2 被称为神经发生的主要调节因子。ZIC2 突变的 hPSC 仍表达多能性标记。然而,它们分化成心肌细胞的能力大大减弱。ZIC2 突变细胞的 RNA-Seq 分析显示,突变体将它们的细胞命运交替转换为非心脏细胞谱系。此外,单细胞 RNA-seq 分析表明,ZIC2 突变体在中胚层形成过程中影响了 apelin 受体相关的信号通路。我们的结果提供了 ZIC2 与人类心脏发生之间的新联系,并记录了全基因组无偏 CRISPR 敲除筛选在体外 hPSC 心脏发生过程中确定人类中胚层前体细胞和心脏祖细胞命运的关键步骤的潜在能力。ZIC2 突变细胞的 RNA-Seq 分析显示,突变体将它们的细胞命运交替转换为非心脏细胞谱系。此外,单细胞 RNA-seq 分析表明,ZIC2 突变体在中胚层形成过程中影响了 apelin 受体相关的信号通路。我们的结果提供了 ZIC2 与人类心脏发生之间的新联系,并记录了全基因组无偏 CRISPR 敲除筛选在体外 hPSC 心脏发生过程中确定人类中胚层前体细胞和心脏祖细胞命运的关键步骤的潜在能力。ZIC2 突变细胞的 RNA-Seq 分析显示,突变体将它们的细胞命运交替转换为非心脏细胞谱系。此外,单细胞 RNA-seq 分析表明,ZIC2 突变体在中胚层形成过程中影响了 apelin 受体相关的信号通路。我们的结果提供了 ZIC2 与人类心脏发生之间的新联系,并记录了全基因组无偏 CRISPR 敲除筛选在体外 hPSC 心脏发生过程中确定人类中胚层前体细胞和心脏祖细胞命运的关键步骤的潜在能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号