当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PPAR gamma targeted molecular docking and synthesis of some new amide and urea substituted 1, 3, 4‐thiadiazole derivative as antidiabetic compound
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-03-04 , DOI: 10.1002/jhet.3941 Yogesh Vaishnav 1 , Dhansay Dewangan 1 , Shekhar Verma 1 , Achal Mishra 1 , Alok Singh Thakur 2 , Pranita Kashyap 3 , Santosh Kumar Verma 4
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-03-04 , DOI: 10.1002/jhet.3941 Yogesh Vaishnav 1 , Dhansay Dewangan 1 , Shekhar Verma 1 , Achal Mishra 1 , Alok Singh Thakur 2 , Pranita Kashyap 3 , Santosh Kumar Verma 4
Affiliation
|
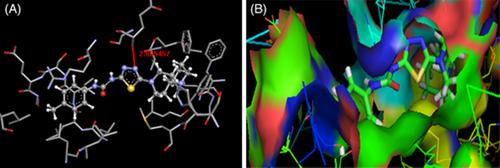
The PPAR‐γ agonist enhances the insulin sensitivity and avoids the disorganized hyperglycemic by promoting the insulin guided cellular uptake of blood glucose. Therefore, in the present work PPAR‐γ has chosen as the target for the molecular docking study to design an effective agonist of the same. By this research work an effort has been made to prepare amide and urea series of 1, 3, 4‐thiadiazole derivatives as 4‐substituted‐N‐(5‐(4‐(1‐piperidino)1‐piperidinyl)‐1,3,4‐(2‐thiadiazolyl)benzamide (4a‐f) and 1‐(4‐substitutedphenyl)‐3‐(5‐(4‐(1‐piperidino)1‐piperidinyl)‐1,3,4‐(2‐thiadiazolyl)urea (6a‐f). Both the docking score as well as the pharmacological animal study data has been suggested that the electron donating group containing compound 4f and 6f are most potent molecules for the antidiabetic activity close to the standard drug pioglitazone. It was further observed that the unsubstituted aromatic ring containing derivatives have also considerable effect (4a and 6a) than the electron withdrawing containing derivatives. After the comparison of biological data for amide and urea series, it was concluded that the urea (6a‐f) series is more effective than the amide series.
中文翻译:

PPARγ靶向分子对接和合成一些新的酰胺和脲取代的1、3、4-噻二唑衍生物作为抗糖尿病化合物
PPAR-γ激动剂通过促进胰岛素引导的细胞对血糖的吸收,提高了胰岛素敏感性,并避免了血糖紊乱。因此,在目前的工作中,PPAR-γ已被选作分子对接研究的目标,以设计有效的激动剂。通过这项研究工作,已努力制备1,4-,3-噻二唑衍生物的酰胺和脲系列,即4-取代-N-(5-(4-(1-(哌啶子基)1-哌啶基)-1,3] ,4-(2-噻二唑基)苯甲酰胺(4a-f)和1-(4-取代苯基)-3-(5-(4-(1-哌啶基)1-哌啶基)-1,3,4-(2-噻二唑基)脲(6a-f)对接评分和药理动物研究数据均提示含电子给体的化合物4f和6f是最有效的抗糖尿病活性分子,接近标准药物吡格列酮。进一步观察到,与含吸电子衍生物相比,含未取代芳环的衍生物也具有相当大的作用(4a和6a)。在比较了酰胺和尿素系列的生物学数据后,得出的结论是,尿素(6a-f)系列比酰胺系列更有效。
更新日期:2020-04-22
中文翻译:

PPARγ靶向分子对接和合成一些新的酰胺和脲取代的1、3、4-噻二唑衍生物作为抗糖尿病化合物
PPAR-γ激动剂通过促进胰岛素引导的细胞对血糖的吸收,提高了胰岛素敏感性,并避免了血糖紊乱。因此,在目前的工作中,PPAR-γ已被选作分子对接研究的目标,以设计有效的激动剂。通过这项研究工作,已努力制备1,4-,3-噻二唑衍生物的酰胺和脲系列,即4-取代-N-(5-(4-(1-(哌啶子基)1-哌啶基)-1,3] ,4-(2-噻二唑基)苯甲酰胺(4a-f)和1-(4-取代苯基)-3-(5-(4-(1-哌啶基)1-哌啶基)-1,3,4-(2-噻二唑基)脲(6a-f)对接评分和药理动物研究数据均提示含电子给体的化合物4f和6f是最有效的抗糖尿病活性分子,接近标准药物吡格列酮。进一步观察到,与含吸电子衍生物相比,含未取代芳环的衍生物也具有相当大的作用(4a和6a)。在比较了酰胺和尿素系列的生物学数据后,得出的结论是,尿素(6a-f)系列比酰胺系列更有效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号