当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One‐pot synthesis of novel polysubstituted furopyran derivatives via pseudo seven‐component reaction (6 + 1) of isocyanides with bisarylidene Meldrum's acid containing ether groups
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-03-03 , DOI: 10.1002/jhet.3949 Elham Moosazadeh 1 , Enayatollah Sheikhhosseini 1 , Dadkhoda Ghazanfari 1 , Shahla Soltaninejad 2
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-03-03 , DOI: 10.1002/jhet.3949 Elham Moosazadeh 1 , Enayatollah Sheikhhosseini 1 , Dadkhoda Ghazanfari 1 , Shahla Soltaninejad 2
Affiliation
|
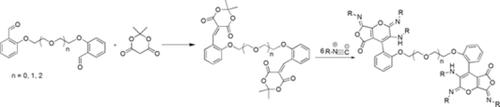
In the present study, a group of polysubstituted furopyran derivatives possessing ether spacer groups were synthesized under good‐to‐exceptional yields via cycloaddition of bisarylidene Meldrum's acid derivatives (1 mmol) with isocyanides (6 mmol) within dichloromethane (CH2Cl2) for 3 to6 hours at room temperature with no assistance from any type of catalysts. The structure of the products was then confirmed by Fourier Transform‐infrared spectroscopy, 1H‐nuclear magnetic resonance spectroscopy, 13C‐nuclear magnetic resonance spectroscopy, and elemental analysis. Moreover, the 5c, 5d, and 5f compounds exhibited favorable pharmaceutical behavior as antibacterial.
中文翻译:

通过异氰酸酯与含醚基的双亚芳基迈德鲁姆酸的假七组分反应(6 + 1)一锅合成新型多取代的呋喃喃衍生物
在本研究中,通过在二氯甲烷(CH 2 Cl 2)中将双亚芳基Meldrum的酸衍生物(1 mmol)与异氰酸酯(6 mmol)环加成,以良好的异常收率合成了一组具有醚间隔基的多取代呋喃吡喃衍生物。室温下3至6个小时,无需任何催化剂的协助。然后通过傅立叶变换红外光谱法,1 H核磁共振光谱法,13 C核磁共振光谱法和元素分析确定产品的结构。此外,5c,5d和5f 化合物表现出良好的抗菌作用。
更新日期:2020-04-22
中文翻译:

通过异氰酸酯与含醚基的双亚芳基迈德鲁姆酸的假七组分反应(6 + 1)一锅合成新型多取代的呋喃喃衍生物
在本研究中,通过在二氯甲烷(CH 2 Cl 2)中将双亚芳基Meldrum的酸衍生物(1 mmol)与异氰酸酯(6 mmol)环加成,以良好的异常收率合成了一组具有醚间隔基的多取代呋喃吡喃衍生物。室温下3至6个小时,无需任何催化剂的协助。然后通过傅立叶变换红外光谱法,1 H核磁共振光谱法,13 C核磁共振光谱法和元素分析确定产品的结构。此外,5c,5d和5f 化合物表现出良好的抗菌作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号