当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theranostic MRI liposomes for magnetic targeting and ultrasound triggered release of the antivascular CA4P.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-04 , DOI: 10.1016/j.jconrel.2020.03.003 Caroline J Thébault 1 , Grégory Ramniceanu 2 , Sarah Boumati 2 , Aude Michel 3 , Johanne Seguin 4 , Benoit Larrat 5 , Nathalie Mignet 4 , Christine Ménager 3 , Bich-Thuy Doan 2
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-04 , DOI: 10.1016/j.jconrel.2020.03.003 Caroline J Thébault 1 , Grégory Ramniceanu 2 , Sarah Boumati 2 , Aude Michel 3 , Johanne Seguin 4 , Benoit Larrat 5 , Nathalie Mignet 4 , Christine Ménager 3 , Bich-Thuy Doan 2
Affiliation
|
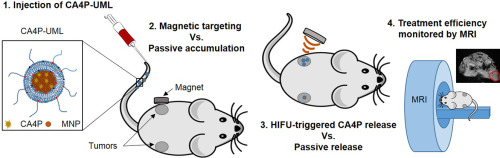
Theranostic nanocarriers of antivascular drug encapsulated in thermosensitive ultramagnetic liposomes can be advantageously designed to provide a locally high concentration and an active delivery, with image-guided Magnetic Resonance Imaging (MRI) so as to reliably cure tumor. We propose a novel therapeutic strategy consisting of the magnetic accumulation of Ultra Magnetic Liposomes (UML) followed by High-Intensity Focused Ultrasound (HIFU) to trigger the release of an antivascular agent monitored by MRI. For this purpose, we co-encapsulated Combretastatin A4 phosphate (CA4P), a vascular disrupting agent, in the core of UML to obtain CA4P-loaded thermosensitive Ultra Magnetic Liposomes (CA4P-UML). To assess the HIFU parameters, the CA4P release has been triggered in vitro by local heating HIFU at the lipids transition temperature. Morphology of endothelial cells was assessed to evaluate the effect of encapsulated versus non-encapsulated CA4P. The efficiency of a treatment combining the magnetic targeting of CA4P-UML with the CA4P release triggered by HIFU was studied in CT26 murine tumors. Tumor perfusion and volume regression parameters were monitored by multiparametric quantitative anatomical and dynamic in vivo MRI at 7 T. Additionally, vascularization and cellularity were evaluated ex-vivo by histology. This thorough investigation showed that the combined treatment exhibited a full benefit. A 150-fold improvement compared with the chemotherapy alone was obtained using a magnetic targeting of CA4P-UML triggered by HIFU, and was consistent with an expected effect on vascularization 24 h after treatment.
中文翻译:

用于磁性靶向和超声的治疗性MRI脂质体触发了抗血管CA4P的释放。
封装在热敏超磁脂质体中的抗血管药物的治疗性纳米载体可以通过图像引导磁共振成像(MRI)进行设计,以提供局部高浓度和主动递送,从而可靠地治愈肿瘤。我们提出了一种新的治疗策略,该方法由超磁性脂质体(UML)的磁性蓄积,然后是高强度聚焦超声(HIFU)来触发MRI监测的抗血管药物的释放。为此,我们在UML的核心中共封装了血管分裂剂Combretastatin A4磷酸酯(CA4P),以获得载有CA4P的热敏超磁脂质体(CA4P-UML)。为了评估HIFU参数,已通过在脂质转变温度下局部加热HIFU在体外触发了CA4P释放。评估内皮细胞的形态,以评估胶囊化与非胶囊化CA4P的效果。在CT26鼠肿瘤中研究了将CA4P-UML的磁性靶向与HIFU触发的CA4P释放相结合的治疗效果。通过多参数定量解剖和动态体内MRI在7 T监测肿瘤灌注和体积消退参数。此外,通过组织学离体评估血管化和细胞形成。这项彻底的调查表明,联合治疗显示出了全部益处。与单纯化疗相比,使用由HIFU触发的CA4P-UML磁性靶向获得了150倍的改善,并且与治疗后24小时对血管形成的预期效果一致。
更新日期:2020-03-04
中文翻译:

用于磁性靶向和超声的治疗性MRI脂质体触发了抗血管CA4P的释放。
封装在热敏超磁脂质体中的抗血管药物的治疗性纳米载体可以通过图像引导磁共振成像(MRI)进行设计,以提供局部高浓度和主动递送,从而可靠地治愈肿瘤。我们提出了一种新的治疗策略,该方法由超磁性脂质体(UML)的磁性蓄积,然后是高强度聚焦超声(HIFU)来触发MRI监测的抗血管药物的释放。为此,我们在UML的核心中共封装了血管分裂剂Combretastatin A4磷酸酯(CA4P),以获得载有CA4P的热敏超磁脂质体(CA4P-UML)。为了评估HIFU参数,已通过在脂质转变温度下局部加热HIFU在体外触发了CA4P释放。评估内皮细胞的形态,以评估胶囊化与非胶囊化CA4P的效果。在CT26鼠肿瘤中研究了将CA4P-UML的磁性靶向与HIFU触发的CA4P释放相结合的治疗效果。通过多参数定量解剖和动态体内MRI在7 T监测肿瘤灌注和体积消退参数。此外,通过组织学离体评估血管化和细胞形成。这项彻底的调查表明,联合治疗显示出了全部益处。与单纯化疗相比,使用由HIFU触发的CA4P-UML磁性靶向获得了150倍的改善,并且与治疗后24小时对血管形成的预期效果一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号