当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Singlet oxygen generation by the reaction of acrolein with peroxynitrite via a 2-hydroxyvinyl radical intermediate.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-03-04 , DOI: 10.1016/j.freeradbiomed.2020.03.003 Leticia C P Gonçalves 1 , Júlio Massari 1 , Saymon Licciardi 2 , Fernanda M Prado 3 , Edlaine Linares 3 , Aline Klassen 1 , Marina F M Tavares 1 , Ohara Augusto 3 , Paolo Di Mascio 3 , Etelvino J H Bechara 2
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-03-04 , DOI: 10.1016/j.freeradbiomed.2020.03.003 Leticia C P Gonçalves 1 , Júlio Massari 1 , Saymon Licciardi 2 , Fernanda M Prado 3 , Edlaine Linares 3 , Aline Klassen 1 , Marina F M Tavares 1 , Ohara Augusto 3 , Paolo Di Mascio 3 , Etelvino J H Bechara 2
Affiliation
|
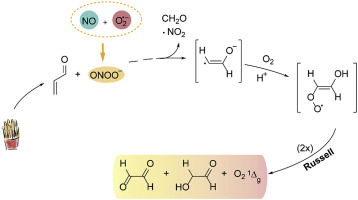
Acrolein (2-propenal) is an environmental pollutant, food contaminant, and endogenous toxic by-product formed in the thermal decomposition and peroxidation of lipids, proteins, and carbohydrates. Like other α,β-unsaturated aldehydes, acrolein undergoes Michael addition of nucleophiles such as basic amino acids residues of proteins and nucleobases, triggering aging associated disorders. Here, we show that acrolein is also a potential target of the potent biological oxidant, nitrosating and nitrating agent peroxynitrite. In vitro studies revealed the occurrence of 1,4-addition of peroxynitrite (k2 = 6 × 103 M-1 s-1, pH 7.2, 25 °C) to acrolein in air-equilibrated phosphate buffer. This is attested by acrolein concentration-dependent oxygen uptake, peroxynitrite consumption, and generation of formaldehyde and glyoxal as final products. These products are predicted to be originated from the Russell termination of •OOCH=CH(OH) radical which also includes molecular oxygen at the singlet delta state (O21Δg). Accordingly, EPR spin trapping studies with the 2,6-nitrosobenzene-4-sulfonate ion (DBNBS) revealed a 6-line spectrum attributable to the 2-hydroxyvinyl radical adduct. Singlet oxygen was identified by its characteristic monomolecular IR emission at 1,270 nm in deuterated buffer, which was expectedly quenched upon addition of water and sodium azide. These data represent the first report on singlet oxygen creation from a vinylperoxyl radical, previously reported for alkyl- and formylperoxyl radicals, and may contribute to better understand the adverse acrolein behavior in vivo.
中文翻译:

丙烯醛与过氧亚硝酸盐通过2-羟基乙烯基自由基中间体反应生成单线态氧。
丙烯醛(2-丙烯醛)是一种环境污染物,食品污染物,是脂质,蛋白质和碳水化合物的热分解和过氧化反应中形成的内源性有毒副产物。像其他α,β-不饱和醛一样,丙烯醛会经历亲核试剂的迈克尔加成反应,例如蛋白质和核碱基的碱性氨基酸残基,从而引发衰老相关疾病。在这里,我们表明丙烯醛也是潜在的生物氧化剂,亚硝化和硝化剂过氧亚硝酸盐的潜在目标。体外研究表明,在空气平衡的磷酸盐缓冲液中,过氧化亚硝酸盐(k2 = 6×103 M-1 s-1,pH 7.2,25°C)1,4-加成反应发生在丙烯醛中。丙烯醛浓度依赖性的氧吸收,过氧亚硝酸盐的消耗以及甲醛和乙二醛作为最终产物的产生证明了这一点。预计这些产物源自•OOCH = CH(OH)基的Russell末端,该基团还包括处于单重态δ态的分子氧(O21Δg)。因此,用2,6-亚硝基苯-4-磺酸根离子(DBNBS)进行的EPR自旋捕集研究揭示了归因于2-羟基乙烯基自由基加合物的6-谱线。单重态氧通过在氘化缓冲液中在1,270 nm处具有特征性的单分子IR发射来鉴定,并有望在加入水和叠氮化钠后淬灭。这些数据代表了关于乙烯基过氧自由基产生单线态氧的第一份报告,以前报道过烷基和甲酰基过氧自由基,并且可能有助于更好地了解体内不良的丙烯醛行为。
更新日期:2020-03-04
中文翻译:

丙烯醛与过氧亚硝酸盐通过2-羟基乙烯基自由基中间体反应生成单线态氧。
丙烯醛(2-丙烯醛)是一种环境污染物,食品污染物,是脂质,蛋白质和碳水化合物的热分解和过氧化反应中形成的内源性有毒副产物。像其他α,β-不饱和醛一样,丙烯醛会经历亲核试剂的迈克尔加成反应,例如蛋白质和核碱基的碱性氨基酸残基,从而引发衰老相关疾病。在这里,我们表明丙烯醛也是潜在的生物氧化剂,亚硝化和硝化剂过氧亚硝酸盐的潜在目标。体外研究表明,在空气平衡的磷酸盐缓冲液中,过氧化亚硝酸盐(k2 = 6×103 M-1 s-1,pH 7.2,25°C)1,4-加成反应发生在丙烯醛中。丙烯醛浓度依赖性的氧吸收,过氧亚硝酸盐的消耗以及甲醛和乙二醛作为最终产物的产生证明了这一点。预计这些产物源自•OOCH = CH(OH)基的Russell末端,该基团还包括处于单重态δ态的分子氧(O21Δg)。因此,用2,6-亚硝基苯-4-磺酸根离子(DBNBS)进行的EPR自旋捕集研究揭示了归因于2-羟基乙烯基自由基加合物的6-谱线。单重态氧通过在氘化缓冲液中在1,270 nm处具有特征性的单分子IR发射来鉴定,并有望在加入水和叠氮化钠后淬灭。这些数据代表了关于乙烯基过氧自由基产生单线态氧的第一份报告,以前报道过烷基和甲酰基过氧自由基,并且可能有助于更好地了解体内不良的丙烯醛行为。









































 京公网安备 11010802027423号
京公网安备 11010802027423号