当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amide-Iminoate Isomerism in Antineuroinflammatory Isoquinoline Alkaloids from Stephania cepharantha.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-03-04 , DOI: 10.1021/acs.jnatprod.9b00483 Jiao Xiao , Jun-Yu Song , Bin Lin , Wei Li 1 , Yan-Qiu Yang 2 , Jing-Yu Liu 2 , Yue Hou 2 , Gang Chen , Ning Li 3
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-03-04 , DOI: 10.1021/acs.jnatprod.9b00483 Jiao Xiao , Jun-Yu Song , Bin Lin , Wei Li 1 , Yan-Qiu Yang 2 , Jing-Yu Liu 2 , Yue Hou 2 , Gang Chen , Ning Li 3
Affiliation
|
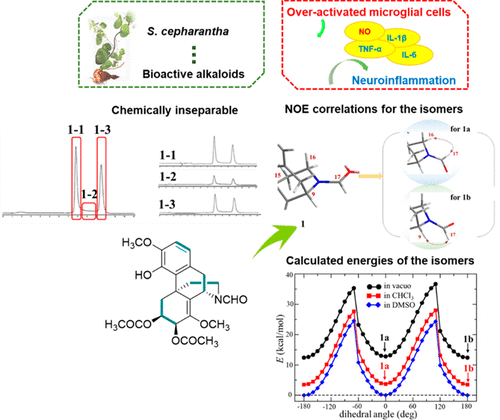
Six new (1-6) and two known (7, 8) alkaloids that were chemically inseparable geometrical isomers (two isomers present in a 1:1 ratio for 1-4 and 6 and a 1:3 ratio for 5, 7, and 8) were identified from Stephania cepharantha. Their structures and absolute configurations were determined by spectroscopic data analyses and comparison of their experimental and calculated ECD spectra. Moreover, using NOE correlations and DFT-based calculations, the NMR data of each geometrical isomer of 1-6 were assigned. The biological evaluation of 1-8 showed that 5 and 6 have stronger inhibitory effects (IC50 values, 12.0 and 12.6 μM, respectively) than minocycline (IC50 value, 17.5 μM) against NO production in overactivated BV2 cells, suggesting they have great potential in the development of neuroinflammatory therapeutics for treating neurodegenerative diseases.
中文翻译:

头孢Stephania的抗神经炎异喹啉生物碱中的酰胺-氨基酸异构体。
六个新(1-6)和两个已知(7、8)生物碱,它们是化学上不可分离的几何异构体(两个异构体的1-4和6的比例为1:1,对于5、7和7的比例为1:3 8)鉴定自Stephania cepharantha。它们的结构和绝对构型是通过光谱数据分析以及实验和计算出的ECD光谱的比较来确定的。此外,使用NOE相关性和基于DFT的计算,分配了1-6个几何异构体的NMR数据。1-8的生物学评估表明,5和6具有比米诺环素(IC50值为17.5μM)更强的抑制作用(分别为IC50值,12.0和12.6μM),对过度活化的BV2细胞产生NO的抑制作用,表明它们具有巨大的潜力。开发用于神经退行性疾病的神经炎性疗法。
更新日期:2020-03-04
中文翻译:

头孢Stephania的抗神经炎异喹啉生物碱中的酰胺-氨基酸异构体。
六个新(1-6)和两个已知(7、8)生物碱,它们是化学上不可分离的几何异构体(两个异构体的1-4和6的比例为1:1,对于5、7和7的比例为1:3 8)鉴定自Stephania cepharantha。它们的结构和绝对构型是通过光谱数据分析以及实验和计算出的ECD光谱的比较来确定的。此外,使用NOE相关性和基于DFT的计算,分配了1-6个几何异构体的NMR数据。1-8的生物学评估表明,5和6具有比米诺环素(IC50值为17.5μM)更强的抑制作用(分别为IC50值,12.0和12.6μM),对过度活化的BV2细胞产生NO的抑制作用,表明它们具有巨大的潜力。开发用于神经退行性疾病的神经炎性疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号