当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthetic Antimicrobial Peptide Tuning Permits Membrane Disruption and Interpeptide Synergy.
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2020-02-21 , DOI: 10.1021/acsptsci.0c00001 Francisco R Fields 1, 2, 3 , Giorgia Manzo 4 , Charlotte K Hind 5 , Jeshina Janardhanan 6 , Ilona P Foik 7 , Phoebe Do Carmo Silva 5 , Rashna D Balsara 6, 8 , Melanie Clifford 5 , Henry M Vu 1, 8 , Jessica N Ross 1, 2 , Veronica R Kalwajtys 1 , Alejandro J Gonzalez 1 , Tam T Bui 9 , Victoria A Ploplis 6, 8 , Francis J Castellino 6, 8 , Albert Siryaporn 7, 10 , Mayland Chang 3, 6 , J Mark Sutton 5 , A James Mason 4 , Shaun Lee 1, 2, 3
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2020-02-21 , DOI: 10.1021/acsptsci.0c00001 Francisco R Fields 1, 2, 3 , Giorgia Manzo 4 , Charlotte K Hind 5 , Jeshina Janardhanan 6 , Ilona P Foik 7 , Phoebe Do Carmo Silva 5 , Rashna D Balsara 6, 8 , Melanie Clifford 5 , Henry M Vu 1, 8 , Jessica N Ross 1, 2 , Veronica R Kalwajtys 1 , Alejandro J Gonzalez 1 , Tam T Bui 9 , Victoria A Ploplis 6, 8 , Francis J Castellino 6, 8 , Albert Siryaporn 7, 10 , Mayland Chang 3, 6 , J Mark Sutton 5 , A James Mason 4 , Shaun Lee 1, 2, 3
Affiliation
|
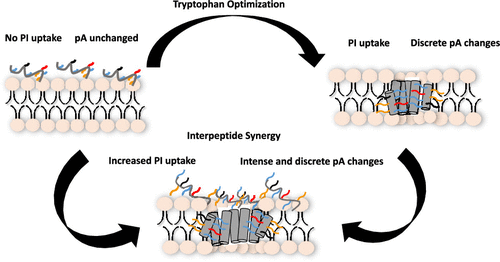
The ribosomally produced antimicrobial peptides of bacteria (bacteriocins) represent an unexplored source of membrane-active antibiotics. We designed a library of linear peptides from a circular bacteriocin and show that pore-formation dynamics in bacterial membranes are tunable via selective amino acid substitution. We observed antibacterial interpeptide synergy indicating that fundamentally altering interactions with the membrane enables synergy. Our findings suggest an approach for engineering pore-formation through rational peptide design and increasing the utility of novel antimicrobial peptides by exploiting synergy.
中文翻译:

合成抗菌肽调整允许膜破坏和肽间协同作用。
核糖体生产的细菌抗菌肽(细菌素)代表了膜活性抗生素的未开发来源。我们从环状细菌素设计了线性肽库,并显示通过选择性氨基酸取代可调节细菌膜中的孔形成动力学。我们观察到抗菌肽间的协同作用,表明从根本上改变与膜的相互作用可以实现协同作用。我们的发现提出了一种通过合理的肽设计来工程化孔形成的方法,并通过利用协同作用来增加新型抗菌肽的实用性。
更新日期:2020-02-21
中文翻译:

合成抗菌肽调整允许膜破坏和肽间协同作用。
核糖体生产的细菌抗菌肽(细菌素)代表了膜活性抗生素的未开发来源。我们从环状细菌素设计了线性肽库,并显示通过选择性氨基酸取代可调节细菌膜中的孔形成动力学。我们观察到抗菌肽间的协同作用,表明从根本上改变与膜的相互作用可以实现协同作用。我们的发现提出了一种通过合理的肽设计来工程化孔形成的方法,并通过利用协同作用来增加新型抗菌肽的实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号