当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of the Convergent, Kilogram-Scale Synthesis of an Antibacterial Clinical Candidate Using Enantioselective Hydrogenation
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-03-04 , DOI: 10.1021/acs.oprd.0c00029 Helen Benson 1 , Karen Bones 2 , Gwydion Churchill 2 , Gair Ford 2 , Lianne Frodsham 2 , Sophie Janbon 1 , Fiona Millington 2 , Lyn Powell 2 , Steven A. Raw 2 , Julie Reid 2 , Andrew Stark 1 , Alan Steven 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-03-04 , DOI: 10.1021/acs.oprd.0c00029 Helen Benson 1 , Karen Bones 2 , Gwydion Churchill 2 , Gair Ford 2 , Lianne Frodsham 2 , Sophie Janbon 1 , Fiona Millington 2 , Lyn Powell 2 , Steven A. Raw 2 , Julie Reid 2 , Andrew Stark 1 , Alan Steven 2
Affiliation
|
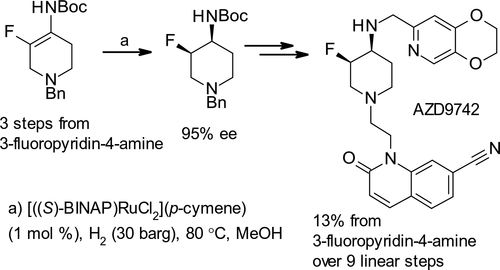
Early chemical development studies into the best way of assembling AZD9742, an antibacterial drug candidate, have involved swapping the order of two reductive aminations. The orthogonally functionalized aminopiperidine partner for these couplings is now enantioselectively synthesized using ruthenium-catalyzed asymmetric hydrogenation. The challenge of controlling defluorination through an appropriate catalyst choice has hitherto prevented this revised sequence from reaching its full potential. However, it is still shown to allow access to the active pharmaceutical ingredient in a stereochemically pure form and has been demonstrated on a multikilogram scale. The reductive aminations in both the original and revised sequences provided different scale-up challenges, and the solutions implemented are described.
中文翻译:

利用对映选择性加氢技术合成,千克级合成抗菌临床候选药物的开发
早期化学开发研究是组装候选抗菌药物AZD9742的最佳方法,涉及交换两个还原性胺基的顺序。现在,使用钌催化的不对称氢化,对映选择性地合成这些偶联的正交官能化氨基哌啶配偶。迄今为止,通过选择合适的催化剂来控制脱氟的挑战已经阻止了该修订后的序列发挥其全部潜力。然而,仍然显示它允许以立体化学纯的形式获得活性药物成分,并且已经在多千克规模上得到了证明。原始序列和修订序列中的还原胺化都提供了不同的放大挑战,并描述了实现的解决方案。
更新日期:2020-04-24
中文翻译:

利用对映选择性加氢技术合成,千克级合成抗菌临床候选药物的开发
早期化学开发研究是组装候选抗菌药物AZD9742的最佳方法,涉及交换两个还原性胺基的顺序。现在,使用钌催化的不对称氢化,对映选择性地合成这些偶联的正交官能化氨基哌啶配偶。迄今为止,通过选择合适的催化剂来控制脱氟的挑战已经阻止了该修订后的序列发挥其全部潜力。然而,仍然显示它允许以立体化学纯的形式获得活性药物成分,并且已经在多千克规模上得到了证明。原始序列和修订序列中的还原胺化都提供了不同的放大挑战,并描述了实现的解决方案。










































 京公网安备 11010802027423号
京公网安备 11010802027423号