当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Pyrido[2,3‐b]indole Derivatives via Rhodium‐Catalyzed Cyclization of Indoles and 1‐Sulfonyl‐1,2,3‐triazoles
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-03-23 , DOI: 10.1002/adsc.201901599 Shengguo Duan 1 , Yuehui An 1 , Bing Xue 1 , Yidian Chen 1 , Wan Zhang 1 , Ze‐Feng Xu 1 , Chuan‐Ying Li 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-03-23 , DOI: 10.1002/adsc.201901599 Shengguo Duan 1 , Yuehui An 1 , Bing Xue 1 , Yidian Chen 1 , Wan Zhang 1 , Ze‐Feng Xu 1 , Chuan‐Ying Li 1
Affiliation
|
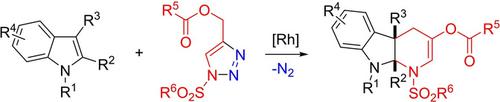
Acyloxy‐substituted α,β‐unsaturated imines generated in situ from triazoles can act as aza‐[4 C] synthons and be trapped by indoles in a stepwise [4 + 2] cycloaddition reaction, thus providing rapid access to valuable pyrido[2,3‐b]indoles in high yields. Attractive features of this reaction system include operational simplicity, readily available substrates, construction of sterically demanding quaternary centers, and convenient derivatization using triflate.
中文翻译:

通过铑催化的吲哚和1-磺酰基1,2,3-三唑的环化反应合成吡啶并[2,3-b]吲哚衍生物
由三唑原位生成的酰氧基取代的α,β-不饱和亚胺可以作为aza- [4 C]合成子,并被吲哚在逐步[4 + 2]环加成反应中捕获,从而提供了快速获取有价值的吡啶基[2, 3‐ b ]吲哚高产。该反应系统的吸引人的特征包括操作简便,易于获得的底物,空间要求严格的四元中心的构建以及使用三氟甲磺酸酯的便捷衍生作用。
更新日期:2020-03-23
中文翻译:

通过铑催化的吲哚和1-磺酰基1,2,3-三唑的环化反应合成吡啶并[2,3-b]吲哚衍生物
由三唑原位生成的酰氧基取代的α,β-不饱和亚胺可以作为aza- [4 C]合成子,并被吲哚在逐步[4 + 2]环加成反应中捕获,从而提供了快速获取有价值的吡啶基[2, 3‐ b ]吲哚高产。该反应系统的吸引人的特征包括操作简便,易于获得的底物,空间要求严格的四元中心的构建以及使用三氟甲磺酸酯的便捷衍生作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号