当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C-H Bond Activation by an Imido Cobalt(III) and the Resulting Amido Cobalt(II) Complex.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-03-27 , DOI: 10.1002/anie.201914718 Alexander Reckziegel 1 , Clemens Pietzonka 1 , Florian Kraus 1 , C Gunnar Werncke 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-03-27 , DOI: 10.1002/anie.201914718 Alexander Reckziegel 1 , Clemens Pietzonka 1 , Florian Kraus 1 , C Gunnar Werncke 1
Affiliation
|
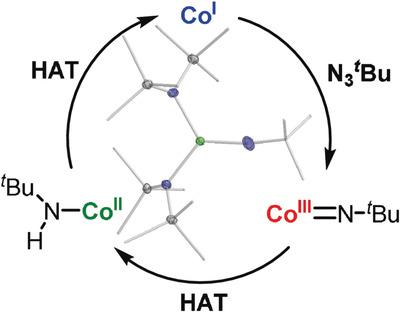
The 3d-metal mediated nitrene transfer is under intense scrutiny due to its potential as an atom economic and ecologically benign way for the directed amination of (un)functionalised C-H bonds. Here we present the isolation and characterisation of a rare, trigonal imido cobalt(III) complex, which bears a rather long cobalt-imido bond. It can cleanly cleave strong C-H bonds with a bond dissociation energy of up to 92 kcal mol-1 in an intermolecular fashion, unprecedented for imido cobalt complexes. This resulted in the amido cobalt(II) complex [Co(hmds)2 (NHt Bu)]- . Kinetic studies on this reaction revealed an H atom transfer mechanism. Remarkably, the cobalt(II) amide itself is capable of mediating H atom abstraction or stepwise proton/electron transfer depending on the substrate. A cobalt-mediated catalytic application for substrate dehydrogenation using an organo azide is presented.
中文翻译:

通过亚氨基钴 (III) 和生成的氨基钴 (II) 络合物激活 CH 键。
3d-金属介导的氮宾转移由于其作为一种原子经济和生态良性方式用于(未)功能化CH键的定向胺化的潜力而受到严格审查。在这里,我们介绍了一种罕见的三角亚氨基钴(III)络合物的分离和表征,该络合物具有相当长的钴亚氨基键。它可以以高达 92 kcal mol-1 的键解离能以分子间方式干净地裂解强 CH 键,这对于亚氨基钴配合物来说是前所未有的。这产生了酰胺基钴(II)络合物[Co(hmds)2 (NHt Bu)]-。该反应的动力学研究揭示了氢原子转移机制。值得注意的是,酰胺钴 (II) 本身能够介导 H 原子夺取或逐步质子/电子转移,具体取决于底物。提出了使用有机叠氮化物进行钴介导的底物脱氢催化应用。
更新日期:2020-03-02
中文翻译:

通过亚氨基钴 (III) 和生成的氨基钴 (II) 络合物激活 CH 键。
3d-金属介导的氮宾转移由于其作为一种原子经济和生态良性方式用于(未)功能化CH键的定向胺化的潜力而受到严格审查。在这里,我们介绍了一种罕见的三角亚氨基钴(III)络合物的分离和表征,该络合物具有相当长的钴亚氨基键。它可以以高达 92 kcal mol-1 的键解离能以分子间方式干净地裂解强 CH 键,这对于亚氨基钴配合物来说是前所未有的。这产生了酰胺基钴(II)络合物[Co(hmds)2 (NHt Bu)]-。该反应的动力学研究揭示了氢原子转移机制。值得注意的是,酰胺钴 (II) 本身能够介导 H 原子夺取或逐步质子/电子转移,具体取决于底物。提出了使用有机叠氮化物进行钴介导的底物脱氢催化应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号