当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CO2 reduction to acetic acid on the greigite Fe3S4{111} surface
Faraday Discussions ( IF 3.3 ) Pub Date : 2020-3-3 , DOI: 10.1039/c9fd00141g David Santos-Carballal 1, 2, 3, 4 , Alberto Roldan 1, 2, 3, 4 , Nora H. de Leeuw 1, 2, 3, 4, 5
Faraday Discussions ( IF 3.3 ) Pub Date : 2020-3-3 , DOI: 10.1039/c9fd00141g David Santos-Carballal 1, 2, 3, 4 , Alberto Roldan 1, 2, 3, 4 , Nora H. de Leeuw 1, 2, 3, 4, 5
Affiliation
|
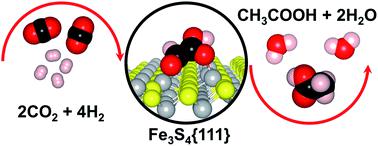
Acetic acid (CH3–COOH) is an important commodity chemical widely used in a myriad of industrial processes, whose production still largely depends on homogeneous catalysts based on expensive rare metals. Here, we report a computational study on the formation of CH3–COOH from carbon dioxide (CO2) as an alternative chemical feedstock on the {111} surface of the low-cost greigite Fe3S4 catalyst. We have used density functional theory calculations with a Hubbard Hamiltonian approach and long-range dispersion corrections (DFT+U−D2) to simulate the various stages of the direct combination of C1 species of different composition to produce glyoxylic acid (CHO–COOH) as a key intermediate in the formation of CH3–COOH. Three reaction mechanisms are considered: (i) the main pathway where the direct formation of the C–C bond takes place spontaneously, followed by a step-wise reduction of CHO–CHOO to CH3–COOH; and the competitive pathways for the non-promoted and H-promoted elimination of hydroxy groups (OH) and water (H2O), respectively from (ii) the carboxyl; and (iii) the carbonyl end of the glyoxylate intermediates. The thermodynamic and kinetic profiles show that the energies for the intermediates on the main pathway are very similar for the two catalytic sites considered, although the activation energies are somewhat larger for the exposed tetrahedral iron (FeA) ion. In most cases, the intermediates for the deoxygenation of the carboxylic acid are less stable than the intermediates on the main pathway, which suggests that the molecule prefers to lose the carbonylic oxygen. The suitable surface properties of the Fe3S4{111} surface show that this material could be a promising sustainable catalyst in future technologies for the conversion of CO2 into organic acid molecules of commercial interest.
中文翻译:

钙铁矿Fe3S4 {111}表面将CO2还原为乙酸
乙酸(CH 3 -COOH)是广泛用于众多工业过程中的重要商品化学品,其生产仍很大程度上取决于基于昂贵的稀有金属的均相催化剂。在这里,我们报告了关于廉价的绢云母Fe 3 S 4催化剂的{111}表面上由二氧化碳(CO 2)形成的CH 3 -COOH作为替代化学原料的计算研究。我们使用了哈伯德哈密顿方法和远程色散校正(DFT + U-D2)的密度泛函理论计算来模拟不同组成的C1物种直接组合以产生乙醛酸(CHO-COOH)的各个阶段,如下所示: CH形成的关键中间体3 –COOH。考虑了三种反应机理:( i)自发形成CC键的主要途径,然后逐步将CHO-CHOO还原为CH 3 -COOH;( ii)从羧基中以非促进和H促进方式分别消除羟基(OH)和水(H 2 O)的竞争途径;( iii)乙醛酸酯中间体的羰基端。热力学和动力学曲线表明,对于所考虑的两个催化位点,主要途径上的中间体的能量非常相似,尽管对于暴露的四面体铁(Fe A) 离子。在大多数情况下,用于羧酸脱氧的中间体比主要途径上的中间体不稳定,这表明该分子更倾向于丢失羰基氧。Fe 3 S 4 {111}表面的合适表面性能表明,这种材料在将来将CO 2转化为具有商业价值的有机酸分子的技术中,可能是有前途的可持续催化剂。
更新日期:2020-03-03
中文翻译:

钙铁矿Fe3S4 {111}表面将CO2还原为乙酸
乙酸(CH 3 -COOH)是广泛用于众多工业过程中的重要商品化学品,其生产仍很大程度上取决于基于昂贵的稀有金属的均相催化剂。在这里,我们报告了关于廉价的绢云母Fe 3 S 4催化剂的{111}表面上由二氧化碳(CO 2)形成的CH 3 -COOH作为替代化学原料的计算研究。我们使用了哈伯德哈密顿方法和远程色散校正(DFT + U-D2)的密度泛函理论计算来模拟不同组成的C1物种直接组合以产生乙醛酸(CHO-COOH)的各个阶段,如下所示: CH形成的关键中间体3 –COOH。考虑了三种反应机理:( i)自发形成CC键的主要途径,然后逐步将CHO-CHOO还原为CH 3 -COOH;( ii)从羧基中以非促进和H促进方式分别消除羟基(OH)和水(H 2 O)的竞争途径;( iii)乙醛酸酯中间体的羰基端。热力学和动力学曲线表明,对于所考虑的两个催化位点,主要途径上的中间体的能量非常相似,尽管对于暴露的四面体铁(Fe A) 离子。在大多数情况下,用于羧酸脱氧的中间体比主要途径上的中间体不稳定,这表明该分子更倾向于丢失羰基氧。Fe 3 S 4 {111}表面的合适表面性能表明,这种材料在将来将CO 2转化为具有商业价值的有机酸分子的技术中,可能是有前途的可持续催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号