当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tumor localization of oncolytic adenovirus assisted by pH-degradable microgels with JQ1-mediated boosting replication and PD-L1 suppression for enhanced cancer therapy.
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-05-06 , DOI: 10.1039/d0bm00172d Haishi Qiao 1 , Xingmei Chen 1 , Qiming Wang 2 , Junmei Zhang 1 , Dechun Huang 1 , Enping Chen 1 , Hongliang Qian 1 , Yinan Zhong 1 , Qi Tang 3 , Wei Chen 1
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-05-06 , DOI: 10.1039/d0bm00172d Haishi Qiao 1 , Xingmei Chen 1 , Qiming Wang 2 , Junmei Zhang 1 , Dechun Huang 1 , Enping Chen 1 , Hongliang Qian 1 , Yinan Zhong 1 , Qi Tang 3 , Wei Chen 1
Affiliation
|
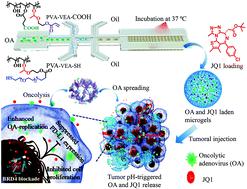
Oncolytic therapy is a fast-developing cancer treatment field based on the promising clinical performance from the selective tumor cell killing and induction of systemic antitumor immunity. The virotherapy efficacy, however, is strongly hindered by the limited virus propagation and negative immune regulation in the tumor microenvironments. To enhance the antitumor activity, we developed injectable pH-degradable PVA microgels encapsulated with oncolytic adenovirus (OA) by microfluidics for localized OA delivery and cancer treatments. PVA microgels were tailored with an OA encapsulation efficiency of 68% and exhibited a pH-dependent OA release as the microgel degradation at mildly acidic conditions. PVA microgels mediated fast viral release and increased replication in HEK293T and A549 cells at a lower pH, and the replication efficiency could be further reinforced by co-loading with one BET bromodomain inhibitor JQ1, inducing significant cytotoxicity against A549 cells. An in vivo study revealed that OA release was highly located at the tumor tissue assisted by PVA microgels, and the OA infection was also enhanced with the addition of JQ1 treatment, meanwhile greatly inhibiting the PD-L1 expression to overcome the immune suppression. OA/JQ1 co-encapsulated injectable microgels exhibited a superior in vivo antitumor activity on the A549 lung tumor-bearing mice by the combination of inhibited proliferation, amplified oncolysis, and potential immune regulation.
中文翻译:

pH降解微凝胶与JQ1介导的增强复制和PD-L1抑制共同辅助溶瘤腺病毒的肿瘤定位,以增强癌症治疗。
溶瘤疗法是一个快速发展的癌症治疗领域,基于选择性杀伤肿瘤细胞和诱导系统性抗肿瘤免疫的有前途的临床表现。然而,病毒的传播受到限制,并且肿瘤微环境中的免疫调节受到负面影响,严重阻碍了病毒疗法的有效性。为了增强抗肿瘤活性,我们通过微流控技术开发了用溶瘤腺病毒(OA)封装的可注射pH降解的PVA微凝胶,用于局部OA递送和癌症治疗。PVA微凝胶经过定制,其OA包封率为68%,并且随着微凝胶在中等酸性条件下的降解而显示出pH依赖性OA释放。在较低pH值下,PVA微凝胶介导了HEK293T和A549细胞中病毒的快速释放和复制的增加,通过与一种BET溴结构域抑制剂JQ1共同加载,可以进一步增强复制效率,从而诱导出对A549细胞的明显细胞毒性。体内研究表明,在PVA微凝胶的辅助下,OA的释放高度位于肿瘤组织上,并且通过加入JQ1处理也可以增强OA感染,同时大大抑制PD-L1的表达以克服免疫抑制作用。OA / JQ1共封装的可注射微凝胶通过抑制增殖,扩增溶瘤作用和潜在的免疫调节作用,对A549肺肿瘤小鼠表现出优异的体内抗肿瘤活性。体内研究表明,在PVA微凝胶的辅助下,OA的释放高度位于肿瘤组织上,并且通过加入JQ1处理也可以增强OA感染,同时大大抑制PD-L1的表达以克服免疫抑制作用。OA / JQ1共封装的可注射微凝胶通过抑制增殖,扩增溶瘤作用和潜在的免疫调节作用,对A549肺肿瘤小鼠表现出优异的体内抗肿瘤活性。体内研究表明,在PVA微凝胶的辅助下,OA的释放高度位于肿瘤组织上,并且通过加入JQ1处理也可以增强OA感染,同时大大抑制PD-L1的表达以克服免疫抑制作用。OA / JQ1共封装的可注射微凝胶通过抑制增殖,扩增溶瘤作用和潜在的免疫调节作用,对A549肺肿瘤小鼠表现出优异的体内抗肿瘤活性。
更新日期:2020-03-03
中文翻译:

pH降解微凝胶与JQ1介导的增强复制和PD-L1抑制共同辅助溶瘤腺病毒的肿瘤定位,以增强癌症治疗。
溶瘤疗法是一个快速发展的癌症治疗领域,基于选择性杀伤肿瘤细胞和诱导系统性抗肿瘤免疫的有前途的临床表现。然而,病毒的传播受到限制,并且肿瘤微环境中的免疫调节受到负面影响,严重阻碍了病毒疗法的有效性。为了增强抗肿瘤活性,我们通过微流控技术开发了用溶瘤腺病毒(OA)封装的可注射pH降解的PVA微凝胶,用于局部OA递送和癌症治疗。PVA微凝胶经过定制,其OA包封率为68%,并且随着微凝胶在中等酸性条件下的降解而显示出pH依赖性OA释放。在较低pH值下,PVA微凝胶介导了HEK293T和A549细胞中病毒的快速释放和复制的增加,通过与一种BET溴结构域抑制剂JQ1共同加载,可以进一步增强复制效率,从而诱导出对A549细胞的明显细胞毒性。体内研究表明,在PVA微凝胶的辅助下,OA的释放高度位于肿瘤组织上,并且通过加入JQ1处理也可以增强OA感染,同时大大抑制PD-L1的表达以克服免疫抑制作用。OA / JQ1共封装的可注射微凝胶通过抑制增殖,扩增溶瘤作用和潜在的免疫调节作用,对A549肺肿瘤小鼠表现出优异的体内抗肿瘤活性。体内研究表明,在PVA微凝胶的辅助下,OA的释放高度位于肿瘤组织上,并且通过加入JQ1处理也可以增强OA感染,同时大大抑制PD-L1的表达以克服免疫抑制作用。OA / JQ1共封装的可注射微凝胶通过抑制增殖,扩增溶瘤作用和潜在的免疫调节作用,对A549肺肿瘤小鼠表现出优异的体内抗肿瘤活性。体内研究表明,在PVA微凝胶的辅助下,OA的释放高度位于肿瘤组织上,并且通过加入JQ1处理也可以增强OA感染,同时大大抑制PD-L1的表达以克服免疫抑制作用。OA / JQ1共封装的可注射微凝胶通过抑制增殖,扩增溶瘤作用和潜在的免疫调节作用,对A549肺肿瘤小鼠表现出优异的体内抗肿瘤活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号