当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
meta-Selective C–H functionalisation of aryl boronic acids directed by a MIDA-derived boronate ester
Chemical Science ( IF 7.6 ) Pub Date : 2020/03/03 , DOI: 10.1039/d0sc00230e Alexander F. Williams 1, 2, 3, 4, 5 , Andrew J. P. White 1, 2, 3, 4, 5 , Alan C. Spivey 1, 2, 3, 4, 5 , Christopher J. Cordier 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2020/03/03 , DOI: 10.1039/d0sc00230e Alexander F. Williams 1, 2, 3, 4, 5 , Andrew J. P. White 1, 2, 3, 4, 5 , Alan C. Spivey 1, 2, 3, 4, 5 , Christopher J. Cordier 1, 2, 3, 4, 5
Affiliation
|
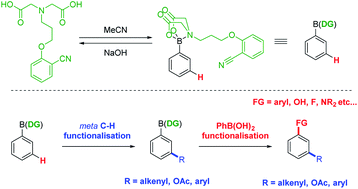
N-Methyliminodiacetic acid (MIDA) boronates are boronic acid derivatives which are stable to reduction, oxidation and transmetalation. This has led to their widespread use as boronic acid protecting groups (PGs) and in iterative cross-couplings. We describe herein the development of a novel MIDA derivative that acts in a dual manner, as a protecting group and a directing group (DG) for meta C(sp2)–H functionalisation of arylboronic acids. Palladium catalysed C–H alkenylations, acetoxylations and arylations are possible, at room temperature and under aerobic conditions. Deprotection to reveal the functionalised boronic acids is rapid and allows for full recovery of the DG. The technique allows the facile diversification of aryl boronic acids and their subsequent use in a range of reactions or in iterative processes.
中文翻译:

由MIDA衍生的硼酸酯指导的芳基硼酸的亚选择性C–H功能化
N-甲基亚氨基二乙酸(MIDA)硼酸盐是硼酸衍生物,对还原,氧化和金属转移稳定。这导致它们广泛用作硼酸保护基(PGs)和迭代交叉偶联。我们在本文中描述了新型MIDA衍生物的发展,该新型MIDA衍生物以双重方式充当meta C(sp 2)的保护基和导向基团(DG))–H芳基硼酸的官能化。在室温和有氧条件下,钯催化的CH链烯基化,乙酰氧基化和芳基化是可能的。脱保护以揭示官能化的硼酸是快速的,并且可以完全回收DG。该技术可以使芳基硼酸易于多样化,并随后用于一系列反应或迭代过程中。
更新日期:2020-03-26
中文翻译:

由MIDA衍生的硼酸酯指导的芳基硼酸的亚选择性C–H功能化
N-甲基亚氨基二乙酸(MIDA)硼酸盐是硼酸衍生物,对还原,氧化和金属转移稳定。这导致它们广泛用作硼酸保护基(PGs)和迭代交叉偶联。我们在本文中描述了新型MIDA衍生物的发展,该新型MIDA衍生物以双重方式充当meta C(sp 2)的保护基和导向基团(DG))–H芳基硼酸的官能化。在室温和有氧条件下,钯催化的CH链烯基化,乙酰氧基化和芳基化是可能的。脱保护以揭示官能化的硼酸是快速的,并且可以完全回收DG。该技术可以使芳基硼酸易于多样化,并随后用于一系列反应或迭代过程中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号